Introduction
Systemic lupus erythematosus (SLE) is a chronic prototypic autoimmune disease characterized by impaired immune tolerance with production of autoantibodies, immune complex formation and dysfunctions of several organs, especially the kidney. Females are more commonly affected [1, 2].
Although the kidney biopsy represents the gold standard for lupus nephritis (LN) diagnosis so far, pathogenesis of LN involves a complicated interaction among genetic, environmental and immune factors [3, 4]. Hence, understanding the cytokine network involved will help to determine substitute non-invasive biomarkers that can monitor renal involvement [5, 6].
Studies suggested that an imbalance between regulatory T cells (Treg), with its associated cytokine interleukin (IL)-35, and T helper 17 (Th17) cells is crucial in immunopathogenesis and progression of multiple autoimmune diseases, particularly SLE [7, 8].
Interleukin 35 is a heterodimeric cytokine, consisting of IL-12A (p35) and Epstein-Barr virus induced 3 (EBI3). It is the newest member of the IL-12 family cytokines which include IL-12, IL-23, and IL-27. The IL-35 receptor is a unique IL-12Rβ2:gp130 heterodimer or homodimer [2, 9-11].
Bioactive IL-35 is secreted by forkhead box protein 3 (Foxp3)+ Treg cells [12, 13], activated B cells [9, 10] and by plasma cells [14].
Upon receptor binding, IL-35 transmits its signals through activation of Janus activating kinase (JAK) family members, then activation and nuclear translocation by members of signal transducer and activator of transcription (STAT). Tregs utilize STAT1 and STAT4 [12], while in primary T cells, recombinant IL-35 (rIL-35) activates STAT1, STAT3, and STAT4 [15].
Its biological functions, which are surprisingly different from its siblings, mainly are assisting differentiation and optimal immunosuppression of Treg cells with conversion of native T cells into an IL-35-producing induced Treg cell population, named iTr35 [9, 11, 13], which directly overwhelms proliferation of effector T (Teff) cells in an antigen-presenting cell (APC)-free culture in vitro [12, 13] and restrains development and function of Th17 cells in vivo, resulting in improvement of collagen-induced arthritis (CIA) [16, 17]. Thus, IL-35 may exert a crucial role in the balance between Th17 cells and Treg cells. In addition, IL-35 facilitates the differentiation of human B cells into Breg cells which secrete IL-35 and IL-10, playing a suppressive role in the regulation of immunity [10, 18].
Thus, IL-35 orchestrates an effective immunosuppressive role in many autoimmune diseases. In rheumatoid arthritis (RA), through the enhanced suppressive function of Tregs and reduced IL-17 by T cells [19], it inhibits angiogenesis [20] and upregulates both p35 and EBI3 subunit proteins in RA synovial membrane [21]. Significant remissions of nephritis and lupus flare were observed upon IL-35 treatment in animal models [22]. Elevated serum levels of IL-35 were reported in systemic sclerosis patients predominantly in those with lung fibrosis [23], polymyositis and dermatomyositis [24], and in psoriatic arthritis compared to psoriasis or healthy controls [25], which in addition to inhibiting inflammation, also inhibits bone erosion by upregulating osteoprotegerin and downregulating the RANK ligand in murine CIA [26].
Plasma levels of IL-35 revealed a significantly positive correlation with platelet counts in active idiopathic thrombocytopenic purpura patients [27]. However, IL-35 was negatively correlated with disease activity of inflammatory bowel disease patients [28]. In primary Sjögren syndrome, serum IL-35 levels were decreased, particularly in active disease [29]. Therefore, this accumulating evidence suggested that IL-35 may serve as a promising future therapeutic agent for autoimmune diseases.
Aim of the study
Immunosuppressive and anti-inflammatory roles of IL-35 in renal disease, especially LN, still need to be clarified, as it may represent a non-invasive biomarker for the diagnosis of LN. To the best of our knowledge, this is the first study aiming to evaluate urine and serum levels of IL-35 in SLE patients with LN and without nephritis, identifying their potential as biomarkers of renal involvement.
Material and methods
In our case control study, 42 patients diagnosed with SLE (age > 18 years) who fulfilled at least 4 of Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) criteria [30], recruited from Internal Medicine and Physical Medicine, Rheumatology and Rehabilitation departments, Ain Shams University Hospital, were compared with 20 healthy age- and gender-matched control subjects. Patients were divided into two groups. Group 1 included 22 patients with evidence of LN as having 24-hour urinary protein (> 0.5 g/day), active urinary sediment and with or without elevated serum creatinine [31], who were scheduled for renal biopsy. Group 2 included 20 SLE patients without any evidence of LN.
Renal biopsy histopathology was classified according to the revised International Society of Nephrology/Renal Pathology Society system (ISN/RPS) [32] and histological activity and chronicity indices were scored [33]. Morning blood samples and first voided midstream urine specimens were collected from SLE patients and controls; for group 1, blood and urine samples were collected on the biopsy day.
Exclusion criteria included: other rheumatic or autoimmune diseases, renal diseases other than LN, infections, diabetes mellitus, malignant tumors, prior treatment with biologic agents, undergoing hemodialysis or with a history of renal transplantation.
All patients were subjected to careful history taking, full clinical examination and laboratory investigations including: complete blood cell count (CBC), erythrocyte sedimentation rate (ESR), antinuclear antibody (ANA), anti-double stranded deoxyribonucleic acid (anti-dsDNA), serum complement (C)3, C4, serum albumin, creatinine and blood urea nitrogen (BUN). A complete urinalysis was performed, and the 24-hour urinary proteins were calculated. SLE disease activity was assessed by the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), which reflects persistent activity in proteinuria, rash, mucous membranes and alopecia [34].
Notably, the patients received oral steroids and the diseasemodifying antirheumatic drugs (DMARDs) hydroxychloroquine, azathioprine, methotrexate, leflunomide, cyclophosphamide and mycophenolate mofetil (MMF).
The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki), the study was approved by the Ethics Committee of the hospital, and informed consent was obtained from subjects participating in the study.
Measurement of serum and urine IL-35 level assay
Peripheral venous blood samples were collected, allowed clotting, centrifuged and sera were stored at –80°C until analysis. Morning urine samples were collected, centrifuged and until analysis the supernatants were stored at –80°C. Serum and urine IL-35 levels were measured using an Enzyme-linked Immunosorbent Assay Kit (Human Interleukin 35 (IL-35) ELISA Kit; Bioassay Technology Laboratory, Shanghai, China) which based on the Biotin double antibody sandwich technology to assay the Human Interleukin-35 (IL-35). To consider variations in urine concentration, urine IL-35 levels were corrected to urine creatinine. Corrected urine IL-35 levels are therefore expressed as picograms per milligram of creatinine (pg/mg Cr).
Statistical analysis
Recorded data were analyzed using IBM SPSS Statistics for Windows, version 20 (IBM Corp., Armonk, N.Y., USA). Shapiro-Wilk test was used to verify normality. Quantitative data were expressed as mean ± standard deviation (SD) while qualitative data were expressed as frequency and percentage. The independent-samples t-test of significance was used when comparing two means, and when comparing between more than two means a one-way analysis of variance (ANOVA) was used. The chi-square (χ2) test of significance was used to compare proportions between qualitative parameters. A post hoc test – least significant difference (LSD) – was used for multiple comparisons between different variables; Pearson’s correlation coefficient (r) test was used to assess the degree of association between two sets of variables, and receiver operating characteristic (ROC) curve analysis was used to find the best cut-off value with detection of sensitivity and specificity at this cut-off value. The confidence interval (CI) was set to 95% and the margin of error accepted was set to 5%. The p-value < 0.05 was considered significant, p < 0.001 was considered highly significant, and p > 0.05 was considered insignificant.
Results
Forty-two SLE patients were divided into 22 patients with LN (20 females and 2 males) and 20 SLE without nephritis (19 females and 1 male) compared with 20 healthy controls (18 females and 2 males) (p = 0.825). No significant difference was detected regarding their age (p = 0.946), as the mean age of the LN group, patients without nephritis and controls was 36.59 ±7.60, 36.60 ±7.78 and 35.90 ±7.55 years, respectively.
Antinuclear antibody and anti-dsDNA antibodies were positive in all patients. There was no significant difference (p > 0.05) in the disease duration and SLEDAI-2K scores between SLE patients with or without nephritis. Patients with LN had significantly higher ESR (p = 0.012), BUN and proteinuria (p < 0.001), and had significantly lower levels of C3 (p = 0.047), C4, hemoglobin and serum albumin (p < 0.001) than did patients without LN (Table 1).
Table 1
Clinical and laboratory characteristics of systemic lupus erythematosus (SLE) patients with lupus nephritis (LN) and without nephritis
[i] Independent sample t-test, p-value > 0.05 non-significant, *p-value < 0.05 significant, **p-value < 0.001 highly significant, LN – lupus nephritis, SLEDAI-2K – Systemic Lupus Erythematosus Disease Activity Index 2000, C3, C4 – complement, ESR – erythrocyte sedimentation rate, WBCs – white blood cells, BUN – blood urea nitrogen
Levels of serum IL-35 were significantly higher (p < 0.001) in patients without LN in comparison to controls and were higher (p < 0.001) in LN patients. Urine IL-35 levels were significantly higher (p < 0.001) in LN patients in comparison to patients without LN and controls but no significant difference regarding urinary IL-35 was observed between patients without LN or controls using the post hoc test (Table 2).
Table 2
Comparison between groups according to serum and urine IL-35 levels
Serum and urine IL-35 in SLE patients with or without LN had a significant correlation (p < 0.001) with ESR (r = 0.770 and 0.866 respectively) and with the SLEDAI-2K score (r = 0.670 and 0.600 respectively). For LN patients, SLEDAI-2K score was correlated positively (p < 0.001) with serum and urine IL-35 (r = 0.677 and 0.806 respectively). In addition, serum and urine IL-35 were significantly inversely correlated (p < 0.001) with serum levels of C3 (r = –0.792 and –0.727 respectively), C4 (r = –0.812 and –0.805 respectively) and hematologic laboratory findings, white blood cells (WBCs) (r = –0.722 and –0.713 respectively), hemoglobin levels (r = –0.803 and –0.889 respectively) and platelets (r = –0.564 and –0.728 respectively).
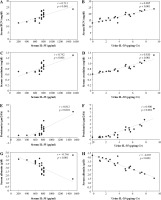
Moreover, in LN patients, a strong correlation (p < 0.001) was observed between serum and urine IL-35 levels with serum BUN, creatinine and proteinuria while serum albumin was significantly inversely correlated (p < 0.001) with them (Fig. 1A-H).
Fig. 1
Correlation of serum and urine IL-35 levels with serum blood urea nitrogen (BUN), creatinine, proteinuria and serum albumin in systemic lupus erythematosus (SLE) patients with lupus nephritis (LN). Significant positive correlations (p < 0.001) of serum and urine IL-35 levels were detected with serum BUN (A, B), serum creatinine (C, D) and proteinuria (E, F) but a significant negative correlation (p < 0.001) with serum albumin (G, H). Pearson’s correlation coefficient (r) test was used and the p-value < 0.001 was highly significant
Renal biopsy of group 1 revealed that 6 cases were class II (mesangial disease), 7 cases were class III (focal proliferative glomerulonephritis), 5 cases were class IV (diffuse proliferative glomerulonephritis) and 4 cases were class V (membranous disease). Histological activity and chronicity indices were 8.73 ±2.41 and 4.91 ±1.64, respectively. Serum IL-35 was significantly correlated with chronicity index (r = 0.659, p = 0.027) but no significant correlation was detected with activity index (r = 0.220, p = 0.516). Urinary IL-35 was not significantly correlated with chronicity or activity index (r = 0.111, 0.407, p = 0.744, 0.215 respectively).
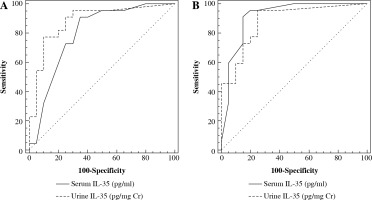
The receiver operating characteristic (ROC) curve showed that area under the curve (AUC) was 0.795 at a serum IL-35 cutoff level > 500 pg/ml and 0.881 at a urine IL-35 cutoff level > 1.098 pg/mg Cr to differentiate LN patients from SLE patients without nephritis (Fig. 2A). To distinguish LN patients from controls, AUC was 0.914 at a serum IL-35 cutoff level > 400 pg/ml and 0.880 at a urine IL-35 cutoff level > 0.8 pg/mg Cr (Fig. 2B).
Fig. 2
Receiver-operating characteristic (ROC) curve for prediction of lupus nephritis (LN) from systemic lupus erythematosus (SLE) patients without nephritis and controls using serum and urine IL-35. A) To distinguish LN patients from SLE patients without nephritis, serum IL-35 has 90% sensitivity and 65% specificity while urine IL-35 has 86.4% sensitivity and 75% specificity. B) To detect LN patients from controls, serum IL-35 has 90.9% sensitivity and 85% specificity while urine IL-35 has 95.5% sensitivity and 75% specificity
Discussion
Lupus nephritis is a devastating manifestation of SLE which involves cytokine dysregulation in its pathogenesis. Hence, a specific cytokine is needed to help early diagnosis, evaluate LN activity and severity and to replace renal biopsy, with its drawbacks [35, 36].
The present study showed that the levels of serum IL-35 were significantly higher in the LN group than in those without LN and controls. Although Ouyang et al. [37] and Álvarez-Rodríguez et al. [38] observed a lower serum IL-35 concentration in patients with SLE, which was comparable with healthy controls, our results were in accordance with Li et al. [39] and Bassiouny et al. [40], who found that the levels of serum IL-35 were significantly higher in patients with SLE than in healthy controls. Similarly, IL-35 levels were reported to be significantly higher in SLE patients [41, 42] which may imply the potential immunosuppressive and anti-inflammatory role of IL-35 in SLE.
To the best of our knowledge, the present study is the first study to assess IL-35 urine levels and showed that the levels of urine IL-35 were significantly higher in the LN group than in with those without LN and controls.
As Treg cells are a major source of IL-35, our results were consistent with Göschl et al. [43] and Bonelli et al. [44], who proved that CD4+CD25-Foxp3+T cells are elevated in SLE patients with renal involvement and in urine sediment samples of patients with active glomerulonephritis and also correlated with the extent of proteinuria. Also, Luk et al. [45] stated that urinary IL-10 mRNA probably comes from Treg cells infiltrating the kidney in LN patients and being leaked into the urine.
Our findings were in accordance with Li et al. [46], who found that human IL-35 is not constitutively expressed in tissues but is expressed in response to inflammatory stimuli, suggesting that the function of human IL-35 is the suppression of full-blown inflammation instead of the prevention of initiation of inflammation.
Also, our data verified that serum and urine IL-35 had a significant association with the SLEDAI-2K scores, which was in accordance with Li et al. [39] and Bassiouny et al. [40], who found that the levels of serum IL-35 were correlated with SLEDAI scores, and suggested that serum IL-35 levels are a highly sensitive and specific biomarker for detection of lupus activity.
Our results were in contrast to Ouyang et al. [37] and He et al. [47], who reported that serum IL-35 levels correlated negatively with SLEDAI-2K. Also, Cai et al. [48] found that there was no significant correlation of plasma IL-35 concentrations with SLEDAI-2K in SLE patients. Major reasons for the discrepancy of these findings may include the heterogeneity of SLE disease and different medical treatments used.
In addition, our study revealed a significant association of serum and urine IL-35 with ESR while it inversely correlated with serum levels of C3 and C4, with hematologic laboratory findings, WBCs, hemoglobin levels and platelets, revealing that serum and urine IL-35 may reflect lupus disease activity.
Our findings were in harmony with Qiu et al. [41], who suggested that increased IL-35 levels may protect immune systems from pathogenic factors to avoid the impairment of tissues and organs, which may reflect a compensatory but inadequate attempt to decrease the inflammatory burden of the disease that may be antagonized by pro-inflammatory factors.
In contrast to He et al. [47], who stated that reduced serum IL-35 levels in LN patients were positively correlated with albumin and were closely related to elevated serum levels of BUN and serum creatinine, serum and urine IL-35 levels in LN patients in our study were significantly inversely correlated with serum albumin, and correlated significantly with BUN and with serum creatinine, suggesting that the presence of IL-35 may show a stronger association not only with acute disease activity but also with the degree of renal insufficiency.
In this study, 24-hour urinary protein values had a strong and significant correlation with serum and urinary IL-35 levels in the group of patients with LN, further indicating that the more severe the renal disease is, the higher are the levels of serum and urinary IL-35.
Our linked findings of elevated serum and urinary IL-35 levels with SLEDAI-2K scores and activity parameters of SLE patients, especially in the LN group, could be explained by the Cai et al. [22] study in which plasma IL-35 levels were elevated, but IL-35 receptor expression in CD4+ Th cells was low and inadequate Treg cell expansion with reduced biological effects could not suppress proinflammatory cytokines in severe SLE patients.
Consequently, Cai et al. [22] postulated that an additional increase in IL-35 levels may restore the balance between proinflammatory and anti-inflammatory cytokines with alleviation of disease flare and supported these findings by demonstrating remission of lupus flare and nephritis in the Murphy Roths Large (MRL)/lpr mice of spontaneous lupus-like disease after administration of recombinant IL-35 treatment accompanied by elevated Treg cells which subsequently enhance the frequency ratio of Treg to Teff cells, increase anti-inflammatory cytokines (IL-10 and IL-2), suppress proinflammatory cytokines [tumor necrosis factor α (TNF-α), IL-6, and IL-17A], and increase expression of IL-35 receptor subunits (gp130 and IL-12Rβ2) with remarkably up-regulated IL-35 receptor-related genes (STAT1 and STAT4), and concluded that IL-35 may act as an efficient therapeutic strategy for SLE.
Furthermore, the study by Hu et al. [49] showed that IL-35 pretreatment could efficiently prevent lipopolysaccharide (LPS)-induced acute kidney injury (AKI) via inhibiting nuclear factor kappa B (NF-κB) activation and reducing pro-inflammatory cytokine production.
Serum IL-35 was significantly correlated with chronicity index of renal biopsy, and this may be explained by Luo et al. [50], who revealed that IL-35 may exert anti-fibrotic effects in kidney fibrosis, as it can reduce extra-cellular matrix aggregation in pulmonary and liver fibrosis through lowering of IL-17 expression and by blocking binding of transforming growth factor β (TGF-β), which is a central profibrotic cytokine in the development of glomerulosclerosis and tubulointerstitial fibrosis, to its receptor.
Our work indicated that the IL-35 biomarker possessed diagnostic value for LN as serum IL-35 had 90% sensitivity and 65% specificity while urine IL-35 had 86.4% sensitivity and 75% specificity to establish the diagnosis of LN in differentiation from SLE patients without nephritis. In addition, to establish the diagnosis of LN in differentiation from healthy individuals, serum IL-35 had 90.9% sensitivity and 85% specificity while urine IL-35 had 95.5% sensitivity and 75% specificity.
Further studies are needed with a larger sample size to detect levels of IL-35 according to the different classes of LN, investigating its expression in renal tissues and identifying effects of different medications to overcome the limitations of our study. Also, further studies are needed to detect evidence about the specificity of IL-35 against other nephropathies or against kidney involvement in other inflammatory diseases such as vasculitis.


