Introduction
Severe allergic reactions occur statistically with a frequency of about 1 case per million administrations of the medical product [1]. In the beginning of 2021, as a response to the COVID-19 pandemic, which was announced by the World Health Organization (WHO) on 11 March 2020, the vaccines, including 2 vaccines based on mRNA technology, 3 viral vector vaccines and 2 inactivated vaccines were conditionally approved [2].
Depending on the source of information, the incidence of anaphylaxis after mRNA vaccines varies between 2.5-4.7 cases per million [3], 4.8–5.1 cases per million [4] and 7.91 cases per million [5], up to 11.1 per million administrations of the vaccine [6]. Preliminary estimates of incidence of anaphylaxis in the USA stand as 11.1 cases per million doses of Pfizer-BioNTech vaccine administered (data for 14–23.12.2020) and 2.5 cases per million doses of Moderna vaccine administered (data for the time period of 21.12.2020–10.01.2021) [7, 8]. According to the report published by The Netherlands Pharmacovigilance Center (Lareb), anaphylaxis with anaphylactic shock occurred the most often after receiving the first dose of the vaccine – Pfizer 75.9% of cases, Moderna 83.3% of cases, following, after the second dose it was 19% of cases (Pfizer) and 5.6% of cases (Moderna). After the third dose, so after the first booster, 5.2% of cases (Pfizer) and 11.1% of cases (Moderna) [9]. According to the report published by CDC (Centers for Disease Control and Prevention), in the period of 14.12–23.12.2020, 21 cases of anaphylactic shock occurred after vaccination with Pfizer BNT162b2 vaccine. Twenty of these patients (95%) were saved, but 1 patient died [8].
Recommendations of the allergological societies concerning COVID-19 vaccination
On account of occurring reports about cases of anaphylaxis after COVID-19 vaccines and growing concerns about safety of the immunisation and risk-benefit ratio, numerous recommendations of allergological societies were published, about ensuring the safety during COVID-19 vaccination.
Experts from the Polish Society of Allergology (PTA) developed two-part guidelines for:
Medical doctors not specializing in allergology, qualifying for COVID-19 vaccinations, with particular attention to emergency equipment supply at every point of vaccination and minimum of 15-minute observation of the patient after vaccination;
Medical doctors specializing in allergology, consulting patients with a medical history of anaphylaxis or severe (uncontrolled) course of allergic diseases, vaccinating against COVID-19 disease – their role in estimation of the probability of occurrence of the severe hypersensitivity after administration of the vaccine. Moreover, in case of significant doubts it is worth consulting a vaccinologist. What is important, in every patient with a medical history of anaphylaxis, especially of unknown cause or multiple cases of anaphylaxis, it is necessary to provide individualised precautions, such as prophylactic provision of intravenous access or prolongation of the time of observation after administration of the vaccine to 30 min [10].
Due to the stand presented by EACCI, no death due to anaphylaxis to COVID-19 vaccines has been confirmed in medical literature. Nevertheless, polyethylene glycol (PEG), polysorbate and tromethamine, which are potential allergens, are also excipients of the vaccine. Authors marked the importance of allergy evaluation of people with the following medical histories: 1 – anaphylaxis to an injectable drug or vaccine containing PEG and derivatives; 2 – anaphylaxis to oral/topical PEG containing products; 3 – recurrent anaphylaxis of unknown cause; 4 – suspected or confirmed allergy to any mRNA vaccine; and 5 – confirmed allergy to PEG or derivatives. If so, it is recommended to perform prick-to-prick skin tests with the left-over solution in the suspected vaccine vial to avoid waste. The prick test panel should also include PEG 4000 or 3500, PEG 2000 and polysorbate 80. The value of the in vitro test is arguable [11].
Potential causes of anaphylaxis
The mechanism causing anaphylaxis by the mRNA vaccines has not been recognised so far and this topic still needs further investigations, nevertheless there are a few hypotheses as described below.
The first hypothesis is that the probable cause of anaphylaxis could be polyethylene glycol (PEG), also named as macrogol, which is an excipient and lipid substance that facilitates transport of nanoparticles containing mRNA into the cells and enabling spike protein synthesis – antigen triggering immunity against SARS-CoV-2 virus [12]. As follows, it is probable that anaphylaxis after COVID-19 vaccine is not associated with the vaccine itself, but with hypersensitivity to excipients. PEG is widely used as a component in the production of drugs, cosmetics and household articles. Patients with hypersensitivity to PEG usually develop recurrent systemic allergic reactions/anaphylaxis before the diagnosis is made.
Moreover, 81% of patients that experienced anaphylaxis after SARS-CoV-2 vaccination, had a prior medical history of allergic reactions to medical products and it is an argument supporting the hypothesis of the allergic cause of anaphylaxis [13]. Based on medical literature, cases of allergy to PEG as an etiologic factor of anaphylaxis after administration of mRNA vaccine suggest an IgE-dependent mechanism [14].
Warren et al. [15] formulated a counterhypothesis based on observation of 11 patients with a medical history of anaphylaxis after mRNA vaccination, none of whom had positive results of skin prick tests with PEG nor detected PEG IgE. Nevertheless, 91% of surveyed people had a positive result of the basophil activation test (BAT) and high PEG IgG antibody titre. Consequently, it was stated that the mechanism of anaphylaxis after administration of mRNA vaccines may be based on pseudoallergy related to activation of the complement system by IgG (CARPA). This hypothesis could explain the fact that patients with positive results of skin prick tests with PEG, with IgE-mediated hypersensitivity, were administered two doses of mRNA vaccine without any allergic reaction.
Unfortunately this subject still needs further investigations. What is more, no uniform strategy to increase safety of COVID-19 vaccination was stated. Different centres developed different strategies, involving administration of premedication, administration of the vaccine in divided doses and also using vaccine based on other technology than mRNA [16].
Potential role and examples of premedication protocols
Based on medical literature, applying premedication does not improve safety of vaccination against COVID-19. Turner et al. [17] did not recommend premedication using H-1 receptor antagonists (antihistamines) or systemic glucocorticosteroids before administration of the COVID-19 vaccine, due to lack of evidence of anaphylaxis prevention. Moreover, premedication with systemic glucocorticosteroids may reduce immunological response to vaccination [18], whereas H1-receptor antagonists may mask first symptoms of a severe allergic reaction [17], leading to delay in diagnosing anaphylaxis. According to Khalid et al. [19], no increased occurrence of allergic reactions in patients without premedication in the form of oral H1-antagonist receptor or leukotriene receptor antagonist was noted.
What is more, a medical case described by Frank et al. [6] proves that, despite premedication including intravenous glucocorticosteroids and diphenhydramine, a 55-year-old woman had a prolonged anaphylaxis reaction after the first dose of Pfizer BNT162b2 vaccine and 5-day hospitalisation was needed with a 73-hour continuous infusion of epinephrine beside the lack of effectiveness of epinephrine in fractionated doses.
On the other hand, Li et al. [16] described the case of a 56-year-old man with anaphylactic reactions after PEG in his past medical history – two episodes, first in 2018 after intra-articular administration of methylprednisolone acetate and second in 2020 after oral administration of PEG3350, who successfully received two doses of mRNA Pfizer-BioNTech vaccine with prior premedication based on 20 mg of cetirizine and 10 mg of montelukast daily given orally for 5 days before the planned vaccination.
The role of skin prick tests in providing safety of the COVID-19 vaccination
Kim et al. [20] summarized that organizations, such as WAO (World Allergy Organisation), ARIA-EAACI (Allergic Rhinitis and its Impact on Asthma – European Academy of Allergy and Clinical Immunology), APAAACI (Asia-Pacific Association of Allergy, Asthma and Clinical Immunology), AAAAI (American Academy of Allergy, Asthma & Immunology), KAAACI (Korean Academy of Asthma, Allergy and Clinical Immunology) recommend providing skin prick tests to patients with a medical history of allergic reactions (other than anaphylaxis) after administration of the first dose of COVID-19 vaccine.
Romantowski et al. [21] described that skin prick tests based on COVID-19 vaccines provide a useful tool that increases safety of vaccination and enables implementation of safe immunization in high-risk patients with positive results of skin prick tests.
During this study, titration was performed according to Nilsson et al. [22] desensitisation protocol: 4 or 5 doses were administered depending on the vaccine volume (0.005; 0.05; 0.1, 0.15 and 0.2 ml if needed), with 15 min time intervals between doses.
What is more, Furman et al. [2] conducted a study on 115 people, divided into 2 groups – 1. group – patients with a hypersensitivity reaction after the first dose of COVID-19 vaccine and 2. group – patients excluded at the vaccination point from administration of COVID-19 vaccine due to anaphylaxis in the past. In all surveyed individuals, the results of skin prick tests with components of the COVID-19 vaccine (Pfizer/BioNTech) were negative. Subsequently, participants were administered the vaccine. No signs of any early allergic reaction in the following 60 min were noticed. One participant reported urticaria that appeared 4–5 h after the vaccination and abated after administration of 20 mg of bilastine. In the second group, skin prick tests and intradermal tests with COVID-19 vaccine compounds were performed and the results were all negative.
Marković et al. [23] conducted skin prick tests in the group of 4 participants, using undiluted vaccine, then an intradermal test, applying dilutions of 1 : 100 and 1 : 10. The results of skin prick tests were negative in all 4 cases, whereas intradermal tests yielded 2 positive (50%) results.
Kruszewski et al. [24] performed a study from 1.05.2021 to 30.09.2021, involving 98 participants who were outpatients of the Department of Infectious Diseases and Allergology, Military Institute of Medicine, National Research Institute, Warsaw, Poland. These patients were admitted for evaluation of the risk of anaphylaxis after administration of COVID-19 vaccine (70 participants) or for clarification whether symptoms suggesting anaphylaxis after the first dose of Comirnaty (Pfizer, USA) vaccine were only due to hypersensitivity to its administration (28 participants). Skin prick tests with the vaccine mentioned above were performed. Only in 2 (2%) cases out of 98, the results were positive. Preliminary experience with skin prick tests using Comirnaty vaccine is a sign that this test is neither sensitive nor specific in prediction and confirmation of the hypersensitivity to its compounds.
In the Department of Infectious Diseases and Allergology, Military Institute of Medicine, National Research Institute, Warsaw, Poland, another study was performed, between 4.06 and 1.10.2021. Skin prick tests using left-overs of Comirnaty vaccine were carried out in the group of 102 participants (85 women and 17 men) with the past medical history of anaphylaxis (after drugs, vaccines, contrast mediums, food, physical effort or unknown causative agent). The results were presented in Table 1 below.
Table 1
Results of the skin prick tests using COVID-19 (Comirnaty) vaccine in participants with a history of immediate hypersensitivity (anaphylaxis) [IHR(A)]
| Variable | Anaphylaxis (Muller grading system) | ||||||
|---|---|---|---|---|---|---|---|
| SPT (–) | SPT(+) | – | I | II | III | IV | |
| 81 patients with a history of IR | 79 | 2 | 0 | ? | ? | 25 | 19 |
| 18 | 9 | 9 | 0 | 0 | |||
| 21 patients with IR after the first dose | 20 | 1 (+/–) | 0 | 0 | 6 | 6 | 9 |
| 5 | 1 | 2 | 0 | 0 | |||
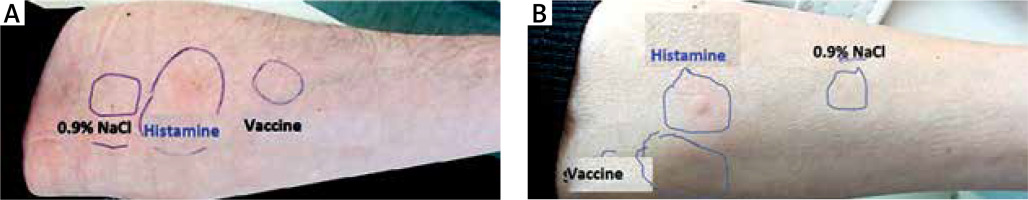
According to the European Network on Drug Allergy (ENDA), the results of the skin prick tests were interpreted after 20 min of each prick of the skin, recognizing presence of the blister with a diameter no less than 3 mm with concomitant erythema as a positive result [24] (Figure 1).
Discussion
Vaccines using mRNA technology contain polyethylene glycol (PEG) that could be responsible for hypersensitivity reactions as described above. Nevertheless, it is worth remembering that the viral vector vaccine contains polysorbate 80 that is structurally similar to PEG and in rare cases constitutes an allergen in the anaphylactic reaction triggered by products and drugs containing polysorbate [25]. Samoliñski et al. [26] emphasized in their publication that anaphylactic shock after contact with inhalant allergens, food, insect venoms is not a contraindication to vaccination and such patients only require special medical supervision (with emergency equipment) and the time of observation should be prolonged. Whereas, a severe anaphylactic reaction after administration of this or any other mRNA vaccine should be treated as an absolute contraindication.
It is worth reminding the most common symptoms of the anaphylactic reaction. They can be divided into several groups – skin symptoms: urticaria, oedema, itching, erythema; respiratory symptoms: rhinitis, voice change, stridor, cough, wheezing, dyspnoea; cardiac system: syncope, loss of consciousness, alteration of the mental state, hypotension and gastrointestinal symptoms: nausea, vomiting, abdominal pain and diarrhoea [25].
There is a number of symptoms that can mimic anaphylaxis – two main groups of such symptoms are vasovagal reactions and reactions due to anxiety syndrome. It is necessary to provide a sample of blood within 4 h after onset of the symptoms to evaluate the serum level of tryptase to perform differential diagnosis. It is worth remembering that an elevated level of serum tryptase confirms anaphylaxis, but normal concentration does not exclude anaphylaxis. Symptoms related to the anxiety syndrome that can be similar to anaphylaxis: stridor, dyspnoea, hypertension, tachycardia, feeling of warmth without redness of the skin, tingling in the oral cavity, face, hands, dizziness, dissociative disorders. In some cases, fragmentary skin redness within the face and neck, nevertheless urticaria and angioedema are absent [25].
Following the CDC (Centers for Disease Control and Prevention) recommendations, the personnel at the vaccination points should be trained in terms of the reaction to anaphylaxis and such a point should be equipped with epinephrine. What is more, prolongation of the observation time from 15 to 30 min is recommended for patients with the past medical history of anaphylaxis regardless of its cause or patients with the history of the immediate allergic reaction after administration of another vaccine or intravenous drugs and also people with contraindications to other COVID-19 vaccines.
If the anaphylaxis is suspected, breathing, circulation and consciousness should be evaluated. The patient should be placed in the supine position. Epinephrine is a drug of first choice, 0.3 mg should be administered intramuscularly, having a memory of maximum dose of 0.5 mg within every 5–15 min [27]. AlMuhizi et al. [28] described successful desensitization with mRNA COVID-19 vaccine in patients with a medical history of anaphylaxis after administration of the first dose of vaccine. The study was performed on a group of 142 participants with an increased risk of allergic reactions after administration of COVID-19 vaccine, in 6 of them desensitization was introduced. All of them were women with the past medical history of allergic diseases – chronic spontaneous urticaria, anaphylaxis after drug or vaccine administration. After completing the desensitization protocol they took the second dose of COVID-19 vaccine without any allergic symptoms.
Invention and approval for use of the COVID-19 vaccines was a ray of hope during the death toll pandemic. Although the preliminary research proved safety of SARS-CoV-2 vaccines, cases of anaphylaxis after administration of the vaccine began to appear. Unfortunately, according to information mentioned above, premedication before the vaccination seems not to provide a positive effect, whereas results of desensitization before the administration of another dose look promising. It is sure that further investigation and research is needed, including allergic reactions after administration of subsequent doses, known as boosters. Identification of the potential mechanisms and risk factors for the adverse reactions would lead to the increase in the safety of vaccination. Patients should be informed that adverse reactions occur rarely, nevertheless they are present. Reliable information enables the patient to make an informed decision, taking into consideration not only benefits of the vaccination, but also potential harm related to adverse reactions and their consequences.
Conclusions
Skin prick tests using mRNA Comirnaty (Pfizer) vaccine seems to be not very sensitive nor specific in prediction and confirmation of the hypersensitivity to its compounds. As a consequence, usability of performing these tests remains under discussion. What is more, despite the contraindications, many patients decided to get the second dose of vaccine with a good effect and no adverse (including allergic) response – such decisions were mainly due to occupational demands or restrictions in border-crossing during COVID-19 pandemic.