Introduction
Vitiligo (VL) is a common pigmentation disorder characterized by the presence of depigmented macules and patches on the skin due to the selective destruction of melanocytes [1, 2]. The exact aetiology of VL has not been still fully understood, but both genetic and environmental factors have been implicated in its pathogenesis [3–5]. Hashimoto’s thyroiditis (HT), conversely, represents an autoimmune disorder wherein the immune system orchestrates the destruction of thyroid glandular cells [6–8]. The coexistence of VL and concomitant HT within the same individual is a frequently encountered phenomenon, with ample research demonstrating a notable correlation between these two clinical entities [9–11]. The management of VL can be challenging, and treatment response varies widely among patients [12]. While some individuals respond well to conventional therapies such as topical corticosteroids, phototherapy, and surgical interventions, others experience limited or no improvement [13]. This inter-individual variability in treatment response has prompted research into potential predictive biomarkers that could aid in personalizing treatment approaches for VL patients. Similarly, the treatment of HT also presents challenges, with some patients achieving remission with standard therapies while others remain refractory [14].
In recent years, there has been growing interest in the role of serum inflammatory factors as potential biomarkers for predicting treatment response in various autoimmune conditions, including VL and concomitant HT [15]. Inflammation is a well-established feature of autoimmune diseases, and the cytokine milieu in the serum of affected individuals may provide valuable insights into disease activity and potential response to treatment [16, 17]. Several studies have investigated the relationship between serum inflammatory markers and disease activity in VL and concomitant HT, with varying degrees of emphasis on different cytokines, chemokines, and other inflammatory mediators. However, the potential utility of these serum markers in predicting treatment response in patients with concomitant VL and concomitant HT remains an understudied area, the current understanding of the pathophysiological mechanisms underlying VL and concomitant HT suggests potential interplay between the two conditions, with shared immunogenetic factors and immune dysregulation contributing to their co-occurrence. Given this intricate relationship, it is plausible that serum inflammatory markers could serve as valuable prognostic indicators for treatment outcomes in patients with both VL and concomitant HT.
Material and methods
Study design and participants
This study conducted a retrospective analysis of the clinical data of 67 patients with VL and comorbid HT admitted to our hospital from January 2022 to December 2023. The patients were categorized based on treatment outcomes into a satisfactory treatment response group (n = 33) and an unsatisfactory treatment response group (n = 34). Criteria for enrolling relevant patients in this work included patients diagnosed with HT [18], patients diagnosed with VL [19], age > 18 years. Criteria for excluding included the patients with no significant thyroid enlargement, those with only throat discomfort; patients presenting with palpitations, shortness of breath, electrocardiographic T-wave changes, and concurrent cardiovascular disease; patients diagnosed with thyroid adenoma or menopausal syndrome. In this study, satisfaction scores above 60% were categorized as the satisfactory treatment response group, while scores below 60% were categorized as the unsatisfactory treatment response group. This study protocol was reviewed and approved by the ethic committee of the Hubei Provincial Hospital of Traditional Chinese Medicine. Since this study is a retrospective cohort study and the identification information of patients is anonymous, there is no need for informed consent from patients and their families.
Clinical study
Vitamin B12 and folate: 4 ml of fasting venous blood was collected in the morning after admission. The concentrations of vitamin B12 and folate were determined using chemiluminescent immunoassay. Ferritin: fasting blood samples were sent to the laboratory for ferritin examination, with concentrations determined using radioimmunoassay. Ferritin levels < 20 µg/l were considered deficient. Zinc: peripheral blood samples were collected from fasting patients, and serum zinc levels were determined using atomic absorption spectroscopy. Zinc levels < 58 µmol/l were considered deficient. Rheumatoid factor: the serum was collected for rheumatoid factor expression analysis using latex agglutination assay. Concentrations of rheumatoid factor > 20 IU/ml were considered positive. The Dermatology Life Quality Index (DLQI) comprises 10 questions focusing on the multifaceted impact of skin diseases on patients in the past 7 days, including physiological aspects (such as itching, pain), psychological effects (such as lack of confidence, depression), social activities, interpersonal relationships, occupational limitations (contact dermatitis), family issues (such as impacting caregiving and sexual life), and treatment-related aspects (time, side effects, and economic burden). Each question is scored on a 4-level scale (0, 1, 2, 3).
Data on DLQI, Physician Global Assessment (PGA), nutritional status indicators (vitamin B12, folate, iron, ferritin, zinc), thyroid parameters (thyroid-stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), thyroperoxidase (TPO), thyroglobulin (Tg)), inflammatory factors (C-reactive protein (CRP), tumor necrosis factor-α) (TNF-α), interleukin (IL) 6 (IL-6), IL-8, IL-10), and autoimmune markers (ANA, ds-DNA, Anti-TPO and Anti-Tg, Rheumatoid factor) for both groups of patients were extracted from the electronic medical record system. ANA, ds-DNA, TPO and Tg antibodies were purchased from EUROIMMUN AG, Germany, while TNF-α, IL-6, IL-8, IL-10 antibodies were purchased from Abcam Biological Technology Co., Ltd., USA.
Results
Baseline characteristics
In this study, we investigated the role of baseline characteristics and dermatological assessment in predicting treatment response in patients with VL and concomitant HT (Table 1). A total of 67 patients were included, with 33 demonstrating unsatisfactory treatment response (unsatisfied group) and 34 showing satisfactory treatment response (satisfied group). Baseline characteristics analysis revealed no significant differences in age, duration of VL, body mass index (BMI), serum inflammatory factors levels, or VL Area Scoring Index (VASI). Analysis of DLQI were 45.67 ±6.78 in the unsatisfied group and 43.29 ±5.45 in the satisfied group, yielding a non-significant difference. Similarly, the PGA scores were 4.75 ±0.89 and 4.42 ±0.76 in the unsatisfied and satisfied group, respectively, with a non-significant difference observed between the unsatisfied and satisfied group. In the comparison of nutritional parameters between the unsatisfied and satisfied group in patients with VL and concomitant HT, no statistically significant differences were observed in serum levels of vitamin B12, folate, iron, ferritin, and zinc (Table 1).
Table 1
Demographic characteristics of patients with VL and concomitant HT and their intakes of nutrients and foods
Descriptive data
The serum thyroid parameters were compared between patients with VL and concomitant HT who exhibited unsatisfied and satisfied treatment responses (Table 2). Remarkably, patients in the unsatisfied group demonstrated higher levels of serum TSH, TPO, and Tg compared to those in the satisfied group. However, no significant differences were found in FT3 and FT4 levels between the two groups. In addition, the analysis of autoimmune markers in patients with VL and concomitant HT revealed noteworthy differences between the unsatisfied and satisfied group (Table 2). Patients in the unsatisfied group demonstrated significantly higher levels of ANA, ds-DNA, anti-TPO, anti-Tg, and rheumatoid factor (p < 0.05) compared to the satisfied group.
Table 2
Serum thyroid parameters and autoimmune markers
Inflammatory factors and treatment response
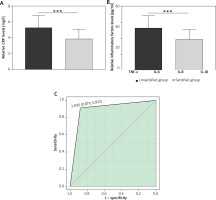
The analysis of inflammatory factors in patients with VL and concomitant HT revealed significant differences between the unsatisfied and satisfied group (Figure 1). Specifically, patients in the unsatisfied group exhibited elevated levels of CRP, TNF-α, IL-6, IL-8, and IL-10 compared to their counterparts in the satisfied group. The correlation analysis revealed significant negative associations between CRP, TNF-α, IL-6, IL-8, and IL-10 levels and treatment response in patients with VL complicated by HT (Table 3). The findings propose the prospective value of these circulating inflammatory biomarkers as predictive indicators for treatment responsiveness within this specific patient cohort. Ultimately, this investigation integrated serum inflammatory biomarkers with predictive potential to formulate a composite model aimed at forecasting the treatment response among individuals afflicted with concurrent VL and concomitant HT. The outcomes revealed that area under curve (AUC) was 0.895 (95% CI: 0.879–0.912), underscoring the robust predictive capacity of the composite serum inflammatory biomarker model (Figure 1).
Table 3
Correlation analysis between serum inflammatory factors and treatment response in patients with vitiligo complicated with Hashimoto’s thyroiditis
| Parameters | r | R2 | P-value |
|---|---|---|---|
| CRP [mg/l] | –0.457 | 0.209 | < 0.001 |
| TNF-α [pg/ml] | –0.43 | 0.185 | < 0.001 |
| IL-6 [pg/ml] | –0.287 | 0.083 | 0.018 |
| IL-8 [pg/ml] | –0.256 | 0.066 | 0.036 |
| IL-10 [pg/ml] | –0.33 | 0.109 | 0.006 |
Figure 1
The predictive value of serum inflammatory factors for treatment response in patients with VL complicated with HT. A – The serum CRP levels of patients with VL and concomitant HT in different groups, ***p < 0.001. B – The serum TNF-α, IL-6, IL-8, IL-10 levels of patients with VL and concomitant HT in different groups, ***p < 0.001, **p < 0.01, *p < 0.05. C – ROC curves of a combined model of serum inflammatory factors score for predicting treatment response in patients with VL complicated with HT, and AUC is 0.895 (95% CI: 0.879–0.912)
Discussion
The co-occurrence of VL and concomitant HT has been well-documented, with numerous studies highlighting a significant association between these two conditions [20]. However, the optimal approach to managing individuals with both VL and concomitant HT poses a substantial challenge due to the varied treatment responses observed among patients. This study’s retrospective analysis of clinical data from 67 patients with comorbid VL and concomitant HT provided a comprehensive evaluation of the potential predictive value of serum inflammatory factors in guiding treatment outcomes. The inclusion of various outcome measures, including dermatological quality of life, nutritional status, thyroid parameters, inflammatory factors, and autoimmune markers, allowed for a multifaceted assessment of the relationship between serum inflammatory markers and treatment response in this patient population.
The baseline characteristics analysis demonstrated the comparability of patients in the unsatisfied and satisfied group, establishing a solid foundation for subsequent research. Despite the lack of significant differences in age, duration of VL, BMI, serum inflammatory factor levels, and VASI between the two treatment response groups, the study proceeded to investigate the potential associations between serum inflammatory factors and treatment response, shedding light on factors beyond the baseline characteristics that may influence treatment outcomes.
Notably, the analysis of serum thyroid parameters yielded compelling findings, indicating a link between elevated levels of TSH, TPO, and Tg and unsatisfactory treatment responses in patients with comorbid VL and concomitant HT. While no significant differences were observed in FT3 and FT4 levels between the unsatisfied and satisfied group, the notable differences in levels of TSH, TPO, and Tg (p < 0.05) suggest a role for thyroid-specific inflammatory markers in predicting treatment response in this patient population.
Furthermore, the assessment of serum inflammatory factors revealed significant differences in levels of CRP and TNF-α, IL-6, IL-8, and IL-10 (p < 0.05) between the unsatisfied and satisfied group, with elevated levels of these inflammatory markers being associated with unsatisfactory treatment responses. These findings highlight the utility of serum inflammatory factors as predictive indicators for treatment response in patients with comorbid VL and concomitant HT, thereby emphasizing the importance of considering inflammatory status in guiding treatment decisions and optimizing patient outcomes.
Importantly, the correlation analysis demonstrated significant negative associations between levels of CRP, TNF-α, IL-6, IL-8, and IL-10 and treatment response in patients with VL complicated by HT (p < 0.05), further underscoring the predictive value of these serum inflammatory factors in guiding clinical decision-making in this patient population. The negative associations identified in the correlation analysis provide compelling evidence of the potential prognostic significance of serum inflammatory markers in predicting treatment responses, lending further support to the role of inflammatory status as a crucial consideration in the management of patients with both VL and concomitant HT. The study showed that the pathogenesis of VL is related to inflammatory factors, such as IL-1, IL-6, and TNF-α [21]. This conclusion supported our view from the side.
The study analysis of serum thyroid parameters showed elevated levels of TSH, TPO, and Tg in the group with unsatisfactory treatment response. Patients in the unsatisfied group exhibited elevated inflammatory factor levels of CRP and TNF-α, IL-6, IL-8, and IL-10 (p < 0.05) compared to their counterparts in the satisfied group. Correlation analysis showed that the levels of the above inflammatory factors were significantly negatively correlated with the treatment response. The construction of a combined model for predicting treatment response using serum inflammatory factors further reinforced the high predictive value of these markers, as evidenced by the substantial AUC obtained. This combined model holds promise for enhancing the precision of treatment approaches in individuals with comorbid VL and concomitant HT, offering a valuable tool for clinicians to tailor interventions based on the predictive value of serum inflammatory factors.
Conclusions
Overall, the findings presented in this manuscript underscore the potential significance of serum inflammatory factors in guiding clinical decision-making and optimizing treatment outcomes for individuals with comorbid VL and concomitant HT. The multifaceted assessment of dermatological quality of life, nutritional status, thyroid parameters, inflammatory factors, and autoimmune markers provided valuable insights into the complex interplay of these conditions and highlighted the potential prognostic value of serum inflammatory markers in predicting treatment responses. While this study offers significant contributions to our understanding of the predictive role of serum inflammatory factors in this patient population, further research is warranted to validate and expand upon these findings, ultimately advancing the personalized management of patients with both VL and concomitant HT.








