Permanent catheters are widely used in haemodialysis of chronic kidney disease. Placement of these catheters is usually through the internal jugular vein or subclavian vein. Catheter placement is associated with risk of inadvertent puncture of the carotid artery, pleura and other nearby structures. These risks have been very much reduced with the use of point of care ultrasound. Even though ultrasound can guide proper puncture of the desired vessel, it cannot help in directing or following the course of the catheter after venepuncture [1]. We report a rare case of a malpositioned dialysis catheter which punctured the roof of the left atrium (LA) after going through the right pulmonary artery (RPA).
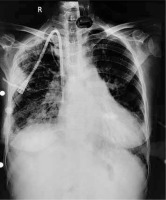
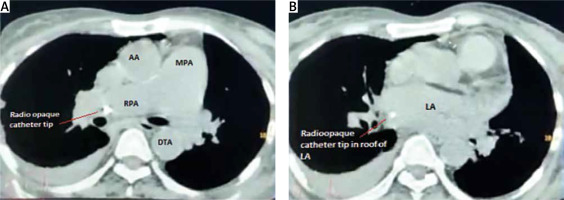
A 51-year-old chronic kidney disease patient with chronic obstructive pulmonary disease underwent catheter insertion with a Hemosplit 14.5 Fr haemodialysis catheter (Bard Access Systems, Utah, USA) through the right subclavian vein in the nephrology unit of our institute. The procedure was conducted with good flow through one port and poor flow through the other port. The catheter guidewire was removed completely but was seen to be coiled on extraction. Some amount of force was also required during the insertion of the catheter as reported by the operator. A post-procedural chest X-ray showed normal position of the catheter. Anticipating that the poor flow through one port could be due to abutment of the catheter against the vessel wall or due to a clot and because of the normal X-ray (Figure 1), the patient was taken for haemodialysis. Two hours into dialysis, the patient became hypotensive with a systolic blood pressure of 70 mm Hg. Dialysis was immediately terminated and imaging in the form of non-contrast neck and chest computed tomography (CT) in view of the patient’s renal status was done. It showed the catheter entering through the right supraclavicular region traversing posterior to the medial end of the clavicle, right subclavian vein, superior vena cava and traversing in the right part of the middle mediastinum. The catheter was seen in close contact with the right pulmonary artery and left atrium with air foci adjacent to the catheter in the left atrium (Figures 2 A, B). As attempts at haemodynamic stabilisation did not succeed, the patient was taken immediately into the operation theatre for exploration by the cardiovascular and thoracic surgery department.
Figure 2
A – Catheter seen in the right pulmonary artery on non-contrast CT chest. B – Catheter seen in the left atrium on non-contrast CT chest
LA – left atrium, MPA – main pulmonary artery, RPA – right pulmonary artery, AA – ascending aorta, DTA – descending thoracic aorta.
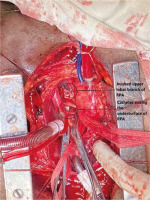
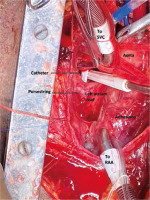
Invasive monitoring lines were taken. Standard midline sternotomy was done with preparations to go on cardiopulmonary bypass (CPB). Pericardiotomy revealed adhesions, hence the pericardium was initially opened minimally so as to allow purse string sutures over the aorta, superior vena cava and right atrium. The aorto-pulmonary septum and branches of the pulmonary artery were dissected. Cardiopulmonary bypass was instituted with aortocavoatrial cannulation as dissection around the pulmonary artery led to bleeding. The catheter tip was seen entering the roof of the left atrium after traversing through the right pulmonary artery (Figure 3). Purse string sutures were taken at the entry point of the catheter into the left atrium with polypropylene 4-0 (26 mm) and one of the two catheter tips was extracted from the LA. On tracing the catheter upwards, it was seen passing through the upper lobar branch of the right pulmonary artery avulsing and shearing it off at its origin and multiple places (Figure 4). The distal end of the avulsed artery was very friable and was in the midst of a group of matted lymph nodes. Due to these findings and given the compromised haemodynamics of the patient, we decided to sacrifice the upper lobar branch. After clamping the RPA, the catheter was extracted completely from the incision site and both ends of the avulsed branch of the RPA were under-run with polypropylene 5-0 (13 mm) sutures such that the RPA continued to supply the rest of the lung. Haemostasis was achieved and the patient was gradually weaned off bypass. The chest was closed over a mediastinal drain. Our total surgical time was 90 minutes with a CPB time of 40 minutes. After observing in the Cardiac ICU for 2 hours, the patient was shifted to the nephrology intensive care unit (ICU) on high ionotropic support of intravenous noradrenaline 0.2 μg/kg/min for further management. However, the patient had a stormy post-operative course complicated by respiratory failure and expired on the third post-operative day in the nephrology ICU. The exact cause of death could not be found as the relatives did not consent to a post mortem examination.
Figure 3
Catheter seen entering left atrial roof
SVC – superior vena cava, RAA – right atrial appendage.
Permacath insertion has early and late complications. Early complications include failed puncture, haemothorax, and wrong cannulation, whereas late complications include infection, catheter thrombosis, venous thrombosis and pneumothorax. A 2019 retrospective study of the complications found a 0.94% incidence of wrong cannulation [2]. Misplacement of dialysis catheters in the accessory hemiazygos, superior intercostal veins and in the brachiocephalic artery and the left internal mammary vein has been reported [3–6]. Thus, there a number of pathways for the malpositioned catheter to gain access. This is the first ever case where a dialysis catheter was found in the left atrium and one which necessitated extraction on cardiopulmonary bypass.
Patients suffering from end stage renal failure and those undergoing dialysis are very high risk candidates for any kind of surgery. Although these patients can safely undergo surgery with careful perioperative management, they still have increased morbidity and mortality rates, especially for emergency open heart operations [7]. We wish to highlight in our case report that our patient had to bear the insult of the misplacement of the catheter after injuring the heart and also that of cardiopulmonary bypass to remove it. Also, the presence of adhesions on opening the pericardium was something which our surgical team did not anticipate. Adhesiolysis was done uneventfully in our case, but it does add to the difficulty in cardiac surgery. Although we managed to keep the time from documentation of the event to surgery, CPB time and surgical time to a minimum, our team could not salvage the patient later in the ICU. This stresses the importance of avoiding such complications even in the best of facilities.
When clinicians perform catheter insertions on critically ill patients, they have to be one hundred percent sure of the completeness of the procedure. Even if an iota of doubt exists, it has to be immediately investigated without relying on assumptions before proceeding further.
Even though entry of a dialysis catheter into a planned vein can be ensured using ultrasound, its subsequent passage cannot be controlled. Ultrasonography is currently being used to detect entry into the internal jugular vein, and fluoroscopy can be used to study the path of the dialysis catheters and hence reduce the complications of catheterization [8]. However, fluoroscopy does not delineate venous structures; hence a catheter which is seen to advance in a correct path may be interpreted as being in the correct position. Even the X-ray in this case was not interpreted as showing a misplaced catheter. Hence it is important that these procedures are performed extremely carefully, especially by younger clinicians, to avoid such untoward complications and preventable morbidity and mortality. Nevertheless, we would still advise ultrasound guided insertion followed by fluoroscopy guidance for placement of such catheters as this strategy minimises the risk of misplacement, perforation and initial malfunction.
In our case, it was possible to perform necessary investigations and immediately take the patient for cardiac surgery as ours is a tertiary care facility. It is important to know that permacath insertions also take place at stand alone dialysis centres where no cardiac surgery facilities are available. For such an event to occur in a non-tertiary setting, the logistical and monetary difficulties that the patient and the treating hospital would have to undergo in order to manage this complication cannot be imagined. Hence it is imperative for nephrologists and cardiac surgeons to be aware of such an untoward complication of permacath insertion and its disastrous consequences so that such events can be avoided in future.