Introduction
Brain tumours can be divided into 2 categories: intra-axial and extra-axial. The position for intra-axial tumours can be either above or below the tentorium. Supratentorial tumours are more common than infratentorial tumours in adults, but not in children. The 3 most prominent infratentorial tumours in children are medulloblastoma, ependymoma, and pilocytic astrocytoma [1–6].
As a non-invasive and non-radiative diagnostic method, magnetic resonance imaging (MRI) is the best imaging modality for assessing brain tumours in children. Moreover, MRI can provide important information regarding correct diagnosis and treatment. Unfortunately, medulloblastoma and ependymoma–the two most common solid tumours located in posterior cranial fossa–might share similar imaging findings even though their treatments and prognoses are different. Therefore, it remains essential to distinguish between these 2 tumour types in clinical practice, especially prior to treatment planning [7–10].
Magnetic resonance spectroscopy (MRS) is an advanced method for investigating the active ingredients inside lesions and is widely used in the treatment of cancerous diseases. Prior knowledge of the concentration of active substances in a tumour can provide valuable information for differential diagnosis, treatment strategy, and prognosis. Several previous studies have used MRS to assess and distinguishing brain tumour types in both adults and children [8, 10–13]. Therefore, in this clinical study, we investigated the impact of MRS parameters on differentiating between paediatric medulloblastoma and ependymoma.
Material and methods
Patient population
The institutional review board of Children’s Hospital 2 approved this prospective study. Informed consent was provided from all patients’ legal representatives before the MRI procedure. The study was conducted at Children’s Hospital 2 over a period of 2 years, beginning in February 2019. A total of 49 patients were included: 40 children with medulloblastoma and 9 children with ependymoma. All patients included in this study underwent an MRI followed by surgery to obtain the histopathological results.
Anaesthesia procedure
The induction of anaesthesia was performed by intravenous injection of midazolam (5 mg/1 mL) at a dose of 0.1 mg/kg (Hameln Pharm GmbH, Germany), followed by propofol 1% anaesthetic (10 mg/1 mL) at a dose of 3 mg/kg (Fresofol, Fresenius Kabi GmbH, Austria).
Magnetic resonance imaging procedure
Paediatric patients were scanned with a 1.5-Tesla MRI machine (Philips, Best, The Netherlands). All paediatric patients were assessed by a multivoxel spectroscopy sequence with the following parameters: TR: 2000 ms; TE: 144 ms; Flip angle: NA; Slice thickness: 15 mm; Gap: NA; Field of view: 120 × 120 mm<sup>2</sup>; Matrix: 10 × 10 mm<sup>2</sup>; Plane: Axial; Number of Acquisitions: 1; Duration: 2.08 minutes. MRS parameters are automatically derived from spectroscopy sequences by utilizing the Spectroview software of Philips IntelliSpace Portal version 11.
Spectroscopy parameters
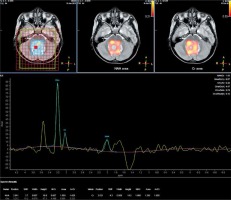
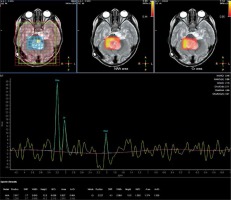
Spectroscopy parameters were comprised of N-acetyl aspartate (NAA), choline (Cho), creatine (Cr), NAA/Cr, choline/creatine (Cho/Cr), and choline/N-acetyl aspartate (Cho/NAA) (Fig. 1, 2).
Data analysis
SPSS software version 26 (IBM Corp, Armonk, New York, USA) was used to perform the statistical analysis. Quantitative variables are presented as the median and interquartile range. Quantitative variables were compared using the U Mann-Whitney test. Receiver operating characteristic (ROC) curve analysis and Youden index were performed to evaluate the cut-off point, accuracy, sensitivity, and specificity of the predictive model. The tests were considered statistically significant at p < 0.05.
Results
The study comprised 49 paediatric patients with posterior cranial fossa tumours (median age = 7 years; male/female ratio = 32/17), including 40 with medulloblastoma (median age = 7 years; male/female ratio = 25/15) and 9 with ependymoma (median age = 3 years; male/female ratio = 7/2).
The Cho, Cho/Cr, and Cho/NAA of medulloblastomas were significantly higher than these of ependymomas (p < 0.05, Table 1).
Table 1
Comparison of magnetic resonance spectroscopy parameters between medulloblastomas and ependymomas
A Cho/NAA cut-off value of 1.24 to predict the diagnosis of medulloblastoma yielded the highest area under the curve (AUC) of 80.3% and the highest sensitivity of 97.5%, while a Cho cut-off value of 4.64 produced the highest specificity value of 88.9% (Table 2, Fig. 3).
Fig. 3
The receiver operating characteristic curves of choline, choline/ creatine, and choline/N-acetyl aspartate
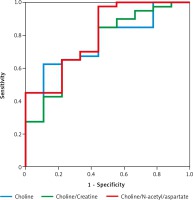
Table 2
Receiver operating characteristic analysis of magnetic resonance spectroscopy parameters for differential diagnosis between medulloblastomas and ependymomas
| Spectroscopy parameters | Cut-off point | AUC | Sensitivity | Specificity | 95% CI interval |
|---|---|---|---|---|---|
| Cho | 4.64 | 0.753 | 0.625 | 0.889 | 0.588–0.918 |
| Cho/Cr | 3.64 | 0.747 | 0.650 | 0.778 | 0.570–0.924 |
| Cho/NAA | 1.24 | 0.803 | 0.975 | 0.556 | 0.640–0.966 |
Discussion
In the current molecular era, recognizing the tumour’s mitotic function and metabolism is crucial for recommending the appropriate therapies. Over the last few years, MRS as a non-invasive and non-ionizing diagnostic method has gained particular importance in the field of paediatric brain tumours. MRS offers more information about the metabolic activity and cellular division of the tumours. Notably, Arle et al. used MRS to evaluate brain tumours in 33 children and observed different values of Cr/NAA, NAA/Cr, and Cr/Cho ratios in PNET, meningeal, and astrocytomas [9]. In a previous study, it was observed that there was an increase in Cho and a decrease in NAA levels in PNET tumours [11]. Similarly, Chang et al. revealed that there was a decrease in NAA level with an increase in the Cho level in paediatric glioblastoma [10]. Moreover, Koob et al. used MRS in the classification and grading of paediatric brain tumours. MRS gave an accuracy of 49.7% in tumour classification and an accuracy of 65.9% in histological subdivisions [8].
Interestingly, Cuellar-Baena et al. also used MRS to study common types of posterior fossa brain tumours in children, including medulloblastoma (n = 8), ependymoma (n = 5), and pilocytic astrocytoma (n = 7). They noted a different spectrum of metabolite values among these 3 groups. The studied metabolites included fatty acids, Cho, leucine, NAA, isoleucine, valine, γ-aminobutyric acid, acetate, glutamate, glutamine, glycine, myoinositol, creatine, serine, phenylalanine, and taurine [12]. In a previous study with MRS with a TE of 30 ms or 136 ms to distinguish between medulloblastoma, ependymoma, haemangioblastoma, and brain metastases, it was also revealed that Cho/NAA ratio helped to distinguish between medulloblastoma and ependymoma with an accuracy of 92% [13]. In this study, Cho and Cho/NAA levels were significantly lower in ependymomas compared to medulloblastoma. Thus, our findings are consistent with previous studies [8–13].
Understanding the genesis and function of both NAA and Cho could help in understanding the role it plays in tumour identification. Theoretically, NAA is synthesized in mitochondria from aspartate and acetyl-Co-A. It is then transported across the mitochondrial membrane and separated into aspartate and acetate by aspartoacylase in the cytoplasm. Therefore, it is suggested that NAA may function as a transporter of acetyl groups across the mitochondrial membrane to produce lipids during development. NAA is mostly concentrated in neurons and is considered a marker for normal neuron function. Brain tumours reduce the number of neurons/axons and reduce cellular density while impairing neuronal function, which often manifests as decreased NAA levels. Meanwhile, Cho concentration is closely related to cell membrane biochemistry, especially cell division. The concentration of Cho is considered a by-product of myelin breakdown. Therefore, when using spectroscopic MRI, cell growth associated with tumour growth may be accompanied by an increase in the Cho level [8–14].
Histopathologically, medulloblastoma is a malignant tumour that is typically characterized by very high cell density and high mitotic activity [15]. In contrast, ependymoma has a lower tumoral grade, lower cell density, and lower cell activity compared to medulloblastoma, which leads to a lower Cho and Cho/NAA ratio [8–14, 16]. Our findings are in line with these previous studies, and confirm that both Cho and Cho/NAA could serve as effective standards in distinguishing between 2 groups of medulloblastoma and ependymoma. We propose a Cho/NAA cut-off value of 1.24 to distinguish between these 2 diseases because the AUC and sensitivity generated exhibited the highest value of 80.3% and 97.5%, respectively. Meanwhile, a Cho cut-off value of 4.64 could be used because it has the highest specificity value of 88.9%.
The small sample size and single-centre nature of the study could be regarded as a limitation of this research. Furthermore, the number of ependymoma patients is relatively low in this study. The researcher only intended to employ MRS to differentiate between paediatric medulloblastoma and ependymoma; therefore, the knowledge of morphological characteristics and conventional MRI features was insufficient. We used multivoxel MRS with TE 144 ms and focused on a few selected substances including NAA, Cho, and Cr. We recommend that further studies with larger sample sizes and multicentre involvement should be performed to validate our findings. These studies should analyse the MRS with TE 35 ms and include a greater number of substances including myo-inositol, glutamate, and lactate. Additionally, future research could also combine both the conventional MRI and MRS to serve as a tool to distinguish between medulloblastoma and ependymoma.
Conclusions
Our findings suggest that Cho and Cho/NAA parameters could serve as differentiating factors between paediatric medulloblastomas and ependymomas. A Cho/NAA cut-off value of 1.24 could predict the diagnosis of medulloblastoma with the highest AUC of 80.3% and sensitivity of 97.5%. Meanwhile, a Cho cut-off value of 4.64 produced the highest sensitivity value of 88.9%. Nonetheless, further research should be conducted to validate these findings.