Introduction
Acute pulmonary embolism (PE) is recognised as an important cause of morbidity and mortality [1]. Depending on the amount of embolic material, patients may present a broad spectrum of symptoms, ranging from sparse to life-threatening. Massive obstruction of pulmonary arteries may lead to right ventricular failure, requiring immediate diagnostic and therapeutic action [2]. In such cases, the purpose of the treatment is to improve pulmonary perfusion by removing or reducing embolic material (reperfusion treatment) and supportive management [3]. Reperfusion treatment includes fibrinolytic therapy, the treatment of choice, percutaneous procedures and surgical treatment.
The indications for surgical embolectomy in the era of thrombolysis have been considerably reduced, and these procedures are currently occasionally performed. However, given the recently published excellent early and long-term results, the more frequent use of surgical embolectomy should be considered [4–6].
Diagnosis
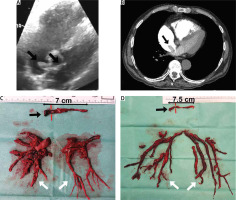
The diagnosis of acute PE is based on clinical symptoms, laboratory and imaging studies, including echocardiography, angiography and computed tomography [7, 8]. With regard to surgery, imaging studies are crucial to assess the embolic material distribution and its surgical accessibility. Echocardiography allows assessment of right ventricular function and detects thrombi in transit in the right heart cavities (Figure 1 A) [9–11]. Pulmonary angiography is an efficient and definitive method for diagnosing acute PE, although it is rarely performed due to low availability and the development of non-invasive diagnostic procedures. Currently, computed tomography pulmonary angiography (CTPA) is the method of choice because of its availability and high-quality imaging of the pulmonary arteries, at least to the level of the segmental branches [12, 13]. It also enables differential diagnosis by enabling the assessment of other thoracic organs.
Current guidelines
According to the 2019 European Society of Cardiology guidelines, the indications for surgical embolectomy are [8]:
A clinical condition, not covered in the guidelines, that increases the risk of death in acute pulmonary embolism is coexisting thrombi in transit in the right heart cavities (Figures 1, 2) [14, 15]. In patients with right ventricular overload, the migration of additional thrombus into the pulmonary circulation may result in a sudden haemodynamic collapse. Surgical treatment should be considered in these cases, as it may provide favourable results [16]. A particular indication for consider surgical treatment is a thrombus wedged in a patent foramen ovale, as other therapies carry the risk of releasing the left atrial portion of the thrombus, which may lead to arterial embolism (Figure 1) [17–19].
Figure 2
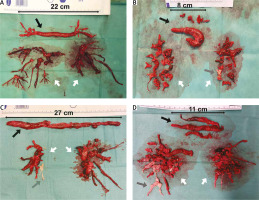
Embolic material removed from the heart cavities and pulmonary arteries (A–D) (black arrow – thrombi removed from right atrium and right ventricle, grey arrow – chronic thromboembolic clots, white arrow – embolic material removed from pulmonary arteries)
As the treatment of PE involves several different options, it is reasonable to consult experts in the field of pulmonary circulation (pulmonary embolism response team, PERT), to choose the best therapeutic option for each patient [20–22].
Surgical treatment
History
The originator of surgical embolectomy was Trendelenburg, who first reported this procedure in 1908 [23]. However, the first successful surgery was performed by Kirschner, a Trendelenburg trainee, in 1924, a few months before the death of his teacher [24]. However, the results continued to be disastrous until Gibbon developed a cardiopulmonary bypass (CPB) in 1953. One incentive for the development of CPB was the poor results of surgical embolectomy, but the first use of CPB in this operation came approximately 10 years later, performed by Cooley in 1961 and Sharp in 1962 [25, 26].
Surgical embolectomy without the use of extracorporeal circulation
Pulmonary artery embolectomy without extracorporeal circulation is now of historical value and can be performed using a mini-thoracotomy or sternotomy. Currently, such procedures are rarely practised; the literature contains descriptions of single cases or small case series [27–30]. However, they are used in selected patients, particularly those in whom heparinisation may increase the risk of bleeding, such as those with cerebrocranial trauma. The operation is based on temporarily clamping the pulmonary artery and aorta to close the blood outflow from the heart. The thrombus is then removed through a small incision in the pulmonary artery. A modification of this method involves additional temporary occlusion of the inferior and superior vena cava to close the blood inflow to the heart. The main disadvantage of this method is the limited processing time and the possibility of removing only large thrombi from the pulmonary artery and its main branches.
Surgical embolectomy using extracorporeal circulation
Without aortic cross-clamping
The surgery is performed through a median sternotomy, and extracorporeal circulation is instituted in a typical way – the aorta and bicaval cannulation [31]. Both venae cavae are snared to isolate the right heart inflow. There is no designated method of surgical access to the pulmonary arteries. Often, similar to surgery without extracorporeal circulation (ECC), only the pulmonary trunk is opened, and embolic material is aspirated and removed with forceps. By enabling better inspection of the pulmonary arteries and removal of embolic material, the right and left pulmonary arteries may also be opened. Various techniques for thrombus evacuation have been described. Embolic material is usually removed with forceps and suction, rarely using Fogarty catheters. In addition, mechanical extrusion of the thrombus is used by manually compressing the lung after opening both pleurae. However, this manoeuvre carries the risk of lung damage and respiratory bleeding [32].
With aortic cross-clamping, normothermia/deep hypothermia with complete circulatory arrest
The procedure can also be performed using ECC with aortic cross-clamping, particularly in patients with coexisting thrombi in the right heart cavities and in the presence of thrombi straddling the foramen ovale. After connecting the extracorporeal circulation, the aorta is cross-clamped, and the administration of cardioplegia stops the heart. All techniques described above can be used for removing embolic material. Retrograde thrombus flushing, described in the literature, is also possible. Through selective pulmonary vein cannulation, blood or saline is injected into the pulmonary veins with access through the atrial septum, and the embolic material is removed through the pulmonary arteriotomy [33–35].
A rarely practised method in surgical embolectomy is the use of moderate to deep hypothermia, with or without total circulatory arrest [36, 37]. Hypothermia significantly slows cell metabolism. Thus, the circulation can safely be totally stopped. Complete circulatory arrest prevents the backflow from bronchial circulation and allows for a bloodless surgical field. The pulmonary trunk is incised above the pulmonary valve towards the left pulmonary artery, whereas the right pulmonary artery is opened from a separate incision between the aorta and the superior vena cava. In this setup, embolic material can be removed, under visual control, from the central and lobar arteries and segmental and subsegmental branches (Figure 2). In addition, it allows one to avoid blind instrumentation within the relatively fragile pulmonary arteries, particularly segmental branches. The thrombus should be very gently separated from the pulmonary artery wall using tweezers and a thin suction device to prevent fragmentation of the embolic material. It also allows for pulmonary endarterectomy, if chronic thromboembolic lesions are found (Figures 2 C, D) [38, 39].
Selection of surgical modality
Currently, there is no recommended method for surgical embolectomy. Recent European guidelines have noted that surgical embolectomy is usually performed with extracorporeal circulation without aortic cross-clamping and cardioplegic cardiac arrest. Nevertheless, the procedures are performed according to the centre’s experience and the surgeon’s preference. Considering the complexity of pulmonary embolism, selecting surgical techniques requires extensive study.
Mechanical circulatory support
Application of ECMO
In recent years, temporary mechanical circulatory support using extracorporeal membrane oxygenation (ECMO) has been successfully practised in critically ill patients with acute PE [40]. However, the existing literature examines case series descriptions only, and thus requires further efficacy evaluation [39, 41, 42]. The use of ECMO in the veno-arterial system can completely replace heart and lung function. Blood from the venous system is collected from the right atrium using a cannula inserted through the femoral vein, and oxygenated blood is pumped through the femoral artery to the arterial system. The use of ECMO in extremely ill patients provides time for implementing the target treatment (including transport to an experienced centre), metabolic compensation or compensation of clotting abnormalities (after ineffective thrombolytic therapy) [43]. It also allows the neurological assessment of patients after cardiac arrest, in situation where this evaluation is often impossible [39, 44]. However, ECMO-related complications, such as bleeding, including the vascular access site, should be considered, particularly in patients undergoing thrombolytic treatment.
ECMO in patients with high-risk acute pulmonary embolism is used as:
Bridge to recovery – with anticoagulant therapy only; however, this management is controversial, and complementary therapy, such as surgical embolectomy, is recommended [45].
Bridging to/supporting target therapy:
Bridge to recovery after surgical embolectomy – in case of surgical treatment failure due to distal pulmonary embolic lesions, coexisting chronic thromboembolic lesions, incomplete embolectomy and right ventricular failure.
Impella RP
The percutaneous right ventricular assist device, Impella RP (Abiomed, Danvers, USA), was approved in 2012 for patients with right ventricular failure following left ventricular assist device implantation, myocardial infarction, heart transplantation or open heart surgery. It is authorised for treating acute PE only in emergencies associated with acute right heart failure or decompensation due to complications of coronavirus disease 2019 (COVID-19). However, in recent years the device has been successfully used in patients with acute PE who are not eligible for conventional treatment. It involves the insertion of a microaxial flow pump with inflow in the inferior vena cava or right atrium and outflow in the pulmonary trunk. The implementation is conditional on adequate gas exchange through the lungs. Reports in the literature are promising, but further studies are required [46–48].
Outcomes of surgical pulmonary embolectomy
Patients undergoing surgical treatment are a relatively heterogeneous group. Among them are haemodynamically unstable patients, in cardiogenic shock, often with multiple organ failures and extreme cases after cardiac arrest. Hence, mortality rates in the literature are highly variable; moreover, with increasing improvements in recent years, considerable differences have been reported over the years. In Karla’s meta-analysis, examining publications over 50 years (1965–2015) with 1579 cases, the overall in-hospital mortality rate was 26.3%, reaching 60% in the early years and dropping to single-digit rates in the best years of the last decade [49]. Subgroup analysis results for pre-2000 and post-2000 showed statistically significant differences in mortality (32.1% vs. 19.0%, respectively). In Kon’s publication, based on the STS Adult Cardiac Surgery Database, evaluating the outcomes of 1,075 surgical embolectomies (SPEs) in North America between 2011 and 2015, the overall mortality rate was 15.9% [50]. Similar results were published in the Japanese Cardiovascular Surgery Database, where the operative mortality rate among 355 patients was 20.6% [51]. Several high-volume, single-centre series (96–136 SPEs) reported excellent treatment outcomes with mortality rates of 4.2–6.6% and showed that centre experience influences the results [52–54].
The outcomes should be considered in the context of the patient’s preoperative condition, with attention to the fact that the surgery is the treatment of last resort for most patients, including a significant percentage of patients (up to 33.9%) who required cardiopulmonary resuscitation (CPR) before surgery [49–51, 55]. Hence, the initial clinical conditions of the patient are the most crucial determinant of surgical outcomes. This finding has been confirmed by several studies, including those based on registry analysis. Kon et al. recorded a mortality rate of 7.9% in the group of patients without shock and 23.7% in the group with shock and not requiring resuscitation, while the mortality rate reached 44.4% among patients with cardiac arrest before the procedure [50]. This relationship is also evident in high-volume centres [54, 56]. The mortality rate in these centres in the submassive/intermediate group is in the range 0–3.6%, in the massive/high-risk group without shock, 0–12%, and in the group of patients requiring CPR, 21–22%. According to Hartman, the mortality rate for patients without the need for CPR was 1.7%, while it was 21.1% (p = 0.03) for patients with pre-procedural cardiac arrest [54]. With these excellent results, there are claims about the underused potential of surgical treatment, encouraging more frequent use of surgical embolectomy in haemodynamically stable patients but at high risk of cardiac decompensation [57].
The management of coexisting thrombi in-transit in the right heart cavities is inconclusive, but many surgical case series demonstrate the possibility of excellent early and long-term outcomes [58, 59]. In the meta-analyses, surgery yields comparable outcomes with those of other methods [16, 60]. However, it should be emphasised that the analysed patients referred for surgical treatment have worse clinical conditions and have a much higher burden of comorbidities. In a systematic literature review of 194 cases, Seo et al. detected statistically significant differences in treatment outcomes in patients with thrombus straggling the PFO [18]. Surgery, versus thrombolysis and anticoagulation, was associated with a lower incidence of post-treatment embolic events (4.5% vs. 14.3% vs. 13.0%; p = 0.044) and lower 60-day mortality (6.3% vs. 35.7% vs. 18.5%; p < 0.001; respectively).
With a few studies, we can determine the long-term outcomes of surgical treatment. The 5-year and 10-year survival rates for hospital-discharged patients were 78%-87% and 66%, respectively [36, 61]. According to Dohle, risk factors for long-term mortality were the absence of deep vein thrombosis (DVT) at the time of acute PE diagnosis (OR = 3.2, CI: 1.2–8.2, p = 0.019) and malignancy (OR = 4.3, CI: 1.8–10.3, p = 0.001).
Comparison with other reperfusion therapies
Surgical embolectomy has a very limited but necessary permanent place in the treatment of acute PE. Hence, numerous studies have compared it with other available modalities. However, no randomised clinical trials have been conducted in this area. The most extensive comparative analysis of surgical embolectomy and fibrinolytic treatment was performed by Lee et al., including 2111 patients from the New York State database hospitalised between 1999 and 2013 [62]. Surgical treatment was applied to only 12% of patients (257 patients), whereas fibrinolytic treatment was administered to 88% of patients (1854 patients). However, it should be noted that the groups differed in several important aspects. Surgically treated patients were characterised by a more frequent history of deep vein thrombosis, pulmonary embolism, congestive heart failure, cerebrovascular disease, or a history of central nervous system ischaemic stroke. Although significant differences were found between the groups, no difference in 30-day mortality (thrombolysis 15.2% vs. SPE 13.2%) was observed. However, thrombolysis was associated with a higher risk of stroke (1.9% vs. 0.8%; p = 0.039) and the need for reintervention (3.8% vs. 1.2%; p = 0.001). At 5-year follow-up, there were no significant differences in survival, with 72.4% in the thrombolysis group and 76.1% in the surgical group. However, there was a higher rate of recurrent acute PE requiring hospital readmission among those treated with fibrinolytic therapy (7.9% vs. 2.8%; p = 0.004). Meanwhile, some single-centre studies favour surgical treatment, although the findings are not statistically significant [63, 64]. It should also be noted that the mortality rate in patients after ineffective fibrinolytic treatment, and thus requiring surgery, is significantly higher than in patients for whom surgical embolectomy was the first-line treatment, reaching approximately 30% [63].
In recent years, catheter-based interventions (CBI) in treating acute PE have become more widespread. However, these are relatively diverse procedures and include fragmentation of embolic material, local thrombolysis, aspiration or mechanical removal [65–67]. They also progressively evolve, making them difficult to compare. Loyalka’s literature review compared outcomes of 1101 SPEs and 1650 CBI [68]. The study reported a mortality rate of 14% in SPE and 5.6% in CBI, but surgical patients were more critically ill; CPR before surgery was required in 21.4% and only 4% of patients undergoing CBI. To this extent, further studies are undoubtedly needed.
Conclusions
Surgical pulmonary embolectomy is a guideline-recommended procedure in selected patients, including salvage therapy for thrombolysis failure. However, the multidisciplinary approach to this disease, combined with careful surgical technique, has reduced mortality in recent years, making surgical embolectomy a safe procedure with a low mortality rate when performed early by experienced teams. Consequently, there is increasing evidence in favour of extending SPE criteria to haemodynamically stable patients with right ventricle dysfunction.