Introduction
Leukocyte adhesion deficiencies (LADs) are a group of primary immunodeficiencies, in which leukocytes, primarily polymorphonuclear leukocytes (PMNs, neutrophils), and monocytes are unable to traffic [1]. Leukocyte trafficking is a dynamic process and involves highly regulated multi-step cascades of interaction between adhesion molecules on leukocytes and endothelium [2].This process is critical for leukocyte extravasation from blood to the sites of inflammation, infection, tissue injury, and for the continuous surveillance of non-self antigens. Impaired leukocyte trafficking in LAD is due to the genetic defect in expression or activation of adhesion molecules. Till date, three types of LAD have been described: type I, II, and III, of which type I is the commonest [3]. Type I LAD results from a congenital defect in the β2 integrin receptor complex CD11/CD18 on the cell surface of leukocytes. The disease is characterized by delayed separation of the umbilical cord at birth, high frequency of omphalitis, recurrent fungal or bacterial infections of skin and mucosa, defect in pus formation and wound healing, marked polymorphonuclear leukocytosis with a neutrophil count ranging from 12,000 to 100,000 cells/mm3, chronic periodontitis, and gingivitis in the adulthood. Type II LAD is very rare form of LAD and is caused by the defect in fucose metabolism resulting in absence of sialyl Lewis X (SLex, CD15s), a fucose-containing glycoconjugate ligand and other fucosylated selectin ligands on neutrophils. Binding of fucosylated selectin ligands to E (CD62E), P (CD62P), and L (CD62L) selectins on the activated endothelium is essential for the rolling phase of adhesion, and this phase is defective in LAD II patients. Fever, leukocytosis, presence of Bombay blood group, growth, and psychomotor retardation are some of the distinct clinical features of LAD II patients. Compared to type I and II, type III LAD has been recently described, and is caused by mutation within the FERMT3gene, which prevents the expression of kindlin-3, a protein expressed by hematopoietic cells, and is important for the regulation of integrin activation. Due to the defect in activation of integrins, LAD III patients display abnormal adhesion and migration of leukocytes, platelet aggregation, and osteoclast functions.
In this report, we present a case of LAD I, who was successfully transplanted with cord blood hematopoietic stem cell at our center, achieving normalization of his immune deficiency.
Case report
A two-and-a-half years old child was born at term, out of non-consanguineous marriage to a primigravidae, by normal delivery. The child was apparently asymptomatic till 18 days of life, when he developed fever following BCG vaccination, and was treated with intravenous antibiotics to which he responded. The umbilical cord separation of the child was on day 18. At two months of age, he had repeated episodes of fever associated with vomiting and left otitis externa. The child was managed conservatively; however, he continued to have recurrent episodes of fever requiring hospitalization. The child had no history of skin infection, gingivitis, or periodontitis. Laboratory investigations revealed a hemoglobin level of 11.2 g/dl, high total leucocyte count (TLC) of 25,200/mm3 with absolute neutrophil count of 22,680/µl (normal range, 1.5-8.5 × 109/l), and absolute lymphocyte count of 2,016/µl (normal range, 3-9.5 × 109/l). Peripheral blood examination was suggestive of polymorphonuclear leukocytosis, with mild left shift and toxic granules. The other hematological and biochemical parameters were normal. Serological tests, which were performed for any infectious etiology including Widal, HIV, anti-HBsAg, anti-HCV, dengue, brucella, EBV, and leptospira were all negative. Additionally, the blood and urine culture did not grow any organism. The child at nine months of age underwent a high resolution computed tomography (HRCT) of head for otitis media, which showed a lobulated soft tissue structure measuring 19.3 × 18.2 mm, suggesting erosion along the inferior aspect of the mastoid and extending up to the tympanic membrane. Magnetic resonance imaging (MRI) revealed an ill-defined area of soft tissue measuring 7.3 × 11 × 6.2 mm adjacent to left temporal bone, extending to lateral margin suggestive of otitis externa. The lesion appeared ulcerated near osseous portion of external auditory canal, associated with swelling and erythema. Tissue biopsy from left ear showed large areas of necrotic tissue with evidence of fat necrosis. Based on these clinical and laboratory findings, a provisional diagnosis of primary immunodeficiency was considered.
Primary immunodeficiency screening workup was performed for the child in our laboratory using flow cytometry, which showed normal T (CD3+), B (CD19+), and NK (CD3–, CD56+) cell frequency with CD4 : CD8 ratio greater than 1.5. The neutrophil oxidative index value of DHR (dihydrorhodamine) assay performed for chronic granulomatous disease was within normal range. However, the LAD I analysis showed markedly reduced surface expression of CD11a/CD18 and CD11b/CD18 markers on gated granulocytes as compared to age-matched healthy control. The frequency of granulocytes having dual expression of CD11a/CD18 and CD11b/CD18 was below 1% (Fig. 1). Based on the results of immunological evaluation, the presence of leukocytosis with neutrophilia, and recurrent infections with persistent otitis media, a definitive diagnosis of LAD I was established.
Fig. 1
Expression of CD11a/CD18 and CD11b/CD18. Peripheral blood mononuclear cells were obtained from whole blood. Cells were stained ex vivo for surface markers, with fluorescently conjugated anti-CD18 PE and anti-CD11a FITC and CD11b PECy5. Samples were acquired with BD FACS Calibur and analyzed using FlowJo software v. 9.810.2. For data analysis, granulocytes were gated on Forward scatter and Side scatter, and cells were checked for the expression of CD11a/CD18 and CD11b/CD18
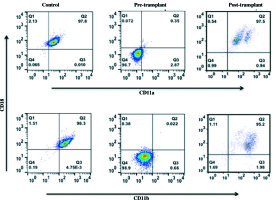
The patient was taken up for matched unrelated umbilical cord blood stem cell transplant, following an extensive pretransplant clinical, laboratory, and radiological workup. He was given non-myeloablative conditioning regimen, with intravenous fludarabine of 30 mg/m2 (total dose, 150 × 0.45 = 67.5 mg) on day –7 and day –4, melphalan of 70 mg/m2on day –3 and day –2 (total dose, 140 × 0.45 = 63 mg), and anti-thymocyte globulin (ATG) of 10 mg/kg on day –4 to day –1 (total dose, 10 × 10 × 4 = 400 mg) [4]. Post-transplant, the child was placed on GVHD prophylaxis with cyclosporine of 1.5 mg/kg i.v. BD from day –1 and mycophenolate mofetil – 30 mg/kg/D in two divided doses w.e.f. D+1. Patient developed febrile neutropenia on day –4 along with grade 2 diarrhea. He was managed with intravenous meropenam, caspofungin, and metronidazole to which he responded. Supportive treatment with antibiotic/antifungal and antiviral prophylaxis was continued. He achieved neutrophil engraftment on D+47 and platelet engraftment on D+48. Presently, the child is healthy at two years post-transplant, and all his laboratory parameters including CD11a/CD18 and CD11b/CD18 expression on granulocytes have normalized (Fig. 1).
Discussion
LAD is a type of immunodeficiency, characterized by the defect in the process of leukocyte adhesion cascade, which includes multiple regulated steps such as rolling, integrin activation, and adhesion of leukocytes. The disease has been categorized into three types, which are summarized in Table 1.
Table 1
An overview of the diagnosis, treatment, clinical, and molecular features of the three types of Leukocyte adhesion deficiency (LAD)
LAD I is a rare autosomal recessive disorder caused by heterogeneous germ-line mutations of CD18 gene located on the long arm of human chromosome 21q22.3, which encodes the β subunit of β2 integrins family [5]. More than 300 cases of LAD I have been documented worldwide and have a very heterogenous molecular nature. A variety of mutations have been observed in patients with LAD I in the CD18 gene, which includes point mutation, splicing defects, deletions, insertion, nonsense, and missense mutations [6]. These mutations result in abnormalities in CD18 protein, which ranges from absence or reduced expression to truncated or unstable protein product formation. Among all the mutations known in CD18 gene, there is a S138P mutation that permits CD11/CD18 expression, but impairs function [7]. Mutations in CD18 interfere with the CD11/CD18 interaction and cause diminished levels of expression of the four integrins on myeloid leukocytes, namely lymphocyte function antigen-1 (LFA-1, CD11a/CD18), Mac-1 (CD11b/CD18), Gp 150/95 (CD11c/CD18), and αDβ2 (CD11d/CD18)[8-11].
Expression of the CD11/CD18 complex on the leukocytes is essential for various activities of leukocyte such as phagocytosis, cell-mediated cytotoxicity, adhesion, and transendothelial migration. In LAD I patients, defect in leukocyte adhesion and migration due to these integrins results in the clinical features, which include susceptibility to recurrent bacterial and fungal infections, elevation of neutrophil counts in the blood during infection, absence of pus at the sites of infection, dystrophic scars from skin injuries, severe gingivitis, and periodontitis [8, 12]. In most cases, the severity of the disease directly correlates with the level of expression of the CD11/CD18 antigens [13]. LAD I patients, categorized as having severe clinical phenotype, would have less than 2% of the normal expression of CD18 and are highly susceptible to systemic infections, which can lead to death in childhood if proper therapeutic intervention is not provided. LAD I patients expressing CD18, which ranges from 2 to 30% of the normal level, would be affected by mild to moderate clinical phenotype and can live to adulthood with careful management, but experiences severe periodontitis, tooth loss, and defect in tissue remodeling of infected sites and wounds. In our patient, the surface expression of CD11a/CD18 and CD11b/CD18 markers on granulocytes was < 1%, associated with severe clinical phenotype.
Disease management depends on the clinical severity. Patients categorized with mild to moderate phenotype are managed with appropriate antibiotics during infection, and by improving oral hygiene in order to prevent periodontitis. However, the only available treatment option for patients with severe LAD I clinical phenotype is hematopoietic stem cell transplantation (HSCT), which has good outcome as was seen in our patient [14, 15]. Recently, Moutsopoulos et al. have reported a successfully disease management of one LAD I patient with ustekinumab, an antibody that blocks the activity of interleukin 23/interleukin 12 and inhibits the production of interleukin 17[16, 17]. The authors have observed resolution of refractory oral inflammation and healing of a severe sacral wound in the LAD patient with ustekinumab treatment. However, treatment of more LAD I patients with this biologic therapy is extremely necessary to understand its use in the disease management.
Here, we wanted to highlight that increased awareness of this rare primary immunodeficiency disease amongst clinicians and availability of simple, robust flow cytometry-based immunological laboratory facilities can help to confirm the diagnosis early in life and prevent fatal complications. Our patient is presently disease-free after HSCT and is on regular follow-up at medical oncology out-patient department (OPD).
Conclusions
LAD I is a rare autosomal recessive primary immunodeficiency disorder caused by mutations in the β subunit of CD18, which manifest in early neonatal period with life-threatening clinical features. Diagnosis often gets delayed because of lack of awareness and specialized laboratory facilities for flow cytometry and genetic studies. Timely diagnosis can prevent from devastating complications affecting patient’s life and can help to plan for HSCT, which is the only potential curative treatment option.


