Introduction
When cancers in women are examined worldwide, breast cancer (BC) is the most common type of cancer (almost ¼ of all cases). Although there have been significant improvements in early diagnosis and treatment in recent years, BC ranks first in mortality among women in relation to the high prevalence (Globocan 2020). Despite the fact that early diagnosis is often associated with a good prognosis, there is currently no biomarker with high sensitivity serving this purpose. Moreover, the detection of a biomarker that may be associated with clinical parameters in case of local disease may play an important role for minimally invasive surgery in the management of both breast and axillary disease.
B7-H3, a recently identified member of the B7 family, is a transmembrane protein broadly expressed in activated T cells, B cells, monocytes, and dendritic cells [1]. Although it was found to stimulate the T cell response and interferon-γ production when initially characterized by Chapoval et al. [2], recent literature considers it to be involved in inhibition of the immune response against cancer [3]. Numerous studies have described B7H3 expression in human malignancies [4–6] and B7H3 appears to play a role in cancer progression, including invasion, migration, and angiogenesis [7]. The proportion of the expression is also associated with a negative prognosis in patients [5]. The role of B7H3 in the immune checkpoint continues to be explored both in terms of understanding the immune pathogenesis of diseases and developing novel immunotherapeutic treatments.
Serum soluble B7H3 (sB7H3) has been previously studied in non-small cell lung cancer (NSCLC) [8], osteosarcoma [9], and hepatocellular cancer [10] and the findings indicated that it may have diagnostic and poor prognostic value. Although there have been studies showing that increased expression of B7H3 on tumor cells in BC patients may be associated with aggressive disease, no study has so far examined the soluble form of B7H3 in BC [4, 11, 12].
In the present study, we investigated the relationship between sB7H3 levels, a very practical biomarker, and clinicopathological variables in BC patients. In order to better demonstrate the potential relationship of B7H3 with the immune system, we included evaluation of stromal tumor-infiltrating lymphocytes (sTILs) in tissue samples in the study. Furthermore, patients with benign breast disease (BBD) (fibroadenoma, etc.) and healthy volunteers were included to evaluate the diagnostic value of sB7H3 in the diagnosis of BC.
Materials and methods
A total of 127 participants were included in this cross-sectional study between January 2020 and September 2021. Ninety-three BC patients with pathological tissue diagnosis, 20 patients with BBD and 14 healthy volunteers as a control group were enrolled. The diagnosis of breast disease (malignant or benign) was established by the same pathologist using Tru-cut biopsy material according to the World Health Organization classification of tumors of the breast 2019 criteria. The detection of clinical T and clinical N was based on magnetic resonance imaging (MRI) and all patients were evaluated by a single radiologist. Blood samples were collected from all BC patients after the diagnosis before they received any treatment. Blood samples were drawn into red capped gel tubes, and were centrifuged at 1000 rpm for 10 minutes. Serum samples were then transferred into microcentrifuge tubes, labeled and stored at –80oC. These samples were analyzed after bringing them to room temperature on the study day. Ethical approval was obtained from the Ethics Committee of the Tekirdağ Namık Kemal University for all procedures before starting the study (ethical approval no: 2019.239.12.14). Written informed consent was obtained from all participants.
Measurement of sB7H3 via ELISA
CD276 (B7H3) levels in serum were measured using the ELISA (enzyme-linked immunosorbent assay) method. A commercial 96-test ELISA kit (Catalog No: CSB-E14285h) from Cusabio (WUHAN HUAWEI BIOTECH CO., LTD. Wuhan, China) was used for this measurement. The properties of the human soluble CD276 kit used in this study are as follows: intra-assay precision, CV 8%; inter-assay precision, CV 10%; measurement range: 3.12–200 ng/ml, and sensitivity: 0.78 ng/ml.
Assessment of tumor-infiltrating lymphocytes
The evaluation of tumor-infiltrating lymphocytes (TIL) was performed on hematoxylin and eosin (H&E) stained slides prepared from thick needle biopsy materials of BC patients, and an Olympus CX41 microscope at x200 magnification was used for this analysis. The area where TIL were evaluated was defined in the stromal region of the tumor according to the TIL evaluation criteria described by the International TIL Working Group 2014 [13]. Tumor margins, carcinoma in situ, normal lobules, tertiary lymphoid structures, polymorphonuclear leukocytes, and areas with crush artefacts and necrosis were excluded from the TIL evaluation. All the slides were evaluated for each case, sTILs were determined and the results were recorded as percentages (%). A detailed explanation of why sTILs were selected for TIL evaluation is described in the Appendix.
Statistical analysis
Normally distributed continuous variables were presented as mean ± standard deviation (SD) and non-normally distributed variables as median ± interquartile range (IQR). Relationships between continuous variables and sB7H3 values were analyzed with the Mann-Whitney U test or the Kruskal-Wallis test. Correlation analyses were tested with Spearman’s rho test. Univariate receiver operating characteristic (ROC) analyses were performed for sB7H3 and other blood-based inflammatory markers used in the diagnosis of BC. Areas under the ROC curve and the sensitivity and specificity at specified points of the curve were determined. Neutrophil-lymphocyte ratio (NLR) was obtained by dividing the absolute neutrophil count by the absolute lymphocyte count, and platelet-lymphocyte ratio (PLR) was obtained by dividing the absolute platelet count by the absolute lymphocyte count. All analyses were carried out using the Statistical Package for Social Sciences, version 23.0 (SPSS, Chicago, IL) ????. Two-tailed p-values < 0.05 were considered statistically significant.
Results
While the mean age of patients with BC was 47 years, it was 44 for patients with BBD and 42 for healthy volunteers. When the clinicopathological variables of BC patients were examined, half of the patients were in the premenopausal period. Estrogen receptor (ER) was negative in 31.1% and progesterone receptor (PR) was negative in 44.4% of the patients. Median ± IQR Ki-67 of all patients was 25 ± 20. Grade 2/3 disease was observed in 93.2% of the patients. When the molecular subtypes were examined, 40 (44%) patients were luminal type, 33 (36.3%) patients were HER2 type, and 18 (19.8%) patients were triple negative. No significant relation was found between sB7H3 and age at the time of diagnosis, menopausal status, ER, PR, Ki67 value, histologic grade, biological subtype, primary tumor status (single vs. multifocal/multicentric), clinical T stage, clinical N stage, tumor diameter on MRI at diagnosis, and sTIL density scores. Demographics and disease characteristics of BC patients and their relationship with sB7H3 levels are presented in detail in Tables 1 and 2.
Table 1
Demographics and disease characteristics of breast cancer patients and their relationship with sB7H3 levels
Table 2
The relationship between sB7H3 and breast cancer subtypes
| Triple-negative | Her2 type | Luminal | p-value | |
|---|---|---|---|---|
| sB7H3 ≥ median* | 6 | 17 | 22 | .298** |
| sB7H3 < median | 12 | 16 | 18 |
sB7H3 levels in BC patients were significantly higher than those in patients with BBD and healthy volunteers (Table 3 vs. Fig. 1). As a confounding factor, the relationship between the age of participants and sB7H3 were also examined. In Spearman’s correlation analysis, no significant relationship was found between sB7H3 and age (p: .631).
Fig. 1
The different sB7H3 levels detected in breast cancer patients, benign breast disease and healthy volunteers (*p < 0.05)
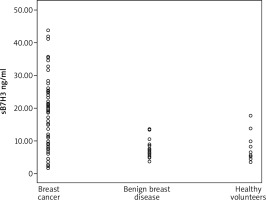
Table 3
sB7H3 levels among patients with breast cancer, patients with benign breast disease and healthy volunteers
| Breast cancer | Benign breast disease | Healthy volunteers | p-value | |
|---|---|---|---|---|
| sB7H3 levels (median ± IQR) | 20.16 ± 17.8* | 7.51 ± 6.65* | 6.49 ± 10.45 | 0.001* |
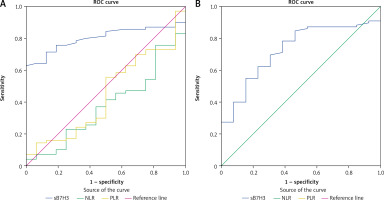
ROC curve analysis results showed that sB7H3 level may be a potential biomarker for distinguishing BC patients from those with BBD (AUC: 0.807; sensitivity: 0.786; specificity: 0.706, Fig. 2 A) and from healthy volunteers (AUC: 0.731; sensitivity: 0.700; specificity: 0.692, Fig. 2 B).
Fig. 2
Diagnostic value of sB7H3, neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for distinguishing breast cancer patients from those with benign breast disease (A), diagnostic value of sB7H3 for distinguishing breast cancer patients from healthy volunteers; receiver operating characteristic curve analysis (B)
Discussion
In this study, we investigated the role of B7H3 levels in BC, which was previously thought to have poor prognostic value in many solid organ tumors. Since it is an easily accessible method in clinical practice, we evaluated serum sB7H3 levels using the ELISA method. sB7H3 values in BC patients were significantly higher than those in patients with BBD and healthy volunteers. As seen in Table 3, and Figures 1 and 2, we found that sB7H3 might be a potential biomarker in distinguishing BC from BBD and healthy volunteers.
Although BC ranks first in terms of both incidence and mortality rate among women worldwide, there is currently no biomarker that is included in guidelines and whose effect in screening or early diagnosis has been demonstrated with randomized studies. Only a limited number of studies have been published showing that blood-based inflammatory parameters such as NLR and PLR may aid in distinguishing BC from BBD and healthy controls [14–16]. As the inhibitory role of B7H1 (PD-L1), the best-known member of the B7 receptor family, in the immune checkpoint has been understood, other members of the B7 family (B7H3, B7H4, etc.) have been the subject of an increasing number of studies in recent years.
Previously, Wang et al. evaluated sB7H3 with the ELISA method, similarly to our study, and examined it in osteosarcoma patients. Benign bone tumors (osteochondroma and bone fibrous dysplasia) and healthy subjects were included as control groups. sB7H3 levels in osteosarcoma patients were significantly higher than in both control groups [9]. In another study, Zhang et al. examined the diagnostic value of sB7H3 in patients with NSCLC. Patients with benign lung disease and healthy subjects were included as control groups. sB7H3 values were significantly higher in NSCLC patients compared to the control groups [8]. In another study, early-stage hepatocellular cancer (ESHCC) patients were compared with cirrhotic patients. sB7H3 similarly achieved significant results in the diagnosis of ESHCC [10]. Until now, the clinical significance of sB7H3 in BC patients has not been studied. In our study, sB7H3 was found to be significantly higher in BC patients than in BBD patients and healthy volunteers. Although NLR and PLR did not reach the level of significance according to ROC analysis, it was found that sB7H3 could be a useful biomarker in clinical practice with its high sensitivity and moderate specificity in the diagnosis of BC when the cut-off value is taken as 8.61 ng/ml.
A study investigating the relationship between B7H3 at the tissue level and clinicopathological variables and prognostic data in BC was conducted by Arigami et al. Specimens from stage 1–3 BC patients and normal breast specimens were compared. B7H3 mRNA levels were evaluated by real-time reverse transcription-polymerase chain reaction and protein expression was evaluated immunohistochemically. B7–H3 mRNA expression was found to correlate with primary tumor size and lymphovascular invasion, with American Joint Committee on Cancer stage of breast cancer, and with the presence and extent of metastasis in axillary sentinel and non-sentinel lymph nodes. Moreover, B7H3 mRNA expression levels were concordant with protein expression [4]. Pizon et al. found that B7H3 and Ki-67, a proliferation marker, correlated with circulating epithelial tumor cells in patients diagnosed with BC [11]. In our study, no significant relationship was found between sB7H3 and clinicopathological variables in BC patients. An important reason for this discrepancy with the current literature may be the inability to perform tissue-based assessment of B7H3 in our study.
Immunotherapy has achieved practice-changing positive results in many solid tumors. In BC patients, triple-negative tumor, PD-L1 positivity and/or increased TIL density are the main factors predicting treatment response. When the potential immunosuppressive effects of B7H3 are taken into account, understanding its relationship with TIL level may be a guide for new immunotherapy combinations. However, results of the studies in the literature on this subject are controversial. In the study conducted by Kim et al. in BC patients, an inverse relationship was detected between pathological B7H3 levels and sTIL density. On the other hand, Altan et al. examined the relationship between B7H3 expression and TIL in NSCLC patients, [17] and Carvajal-Hausdorf et al. in small cell lung cancer patients [18]. In both studies, no correlation was found between B7H3 expression and TIL levels. In the present study, we investigated whether there was a relationship between the sTILs score and sB7H3 values. No significant relationship was found between these two variables in the correlation analysis.
Despite the significant results we obtained in this study, it had serious limitations. It has been reported in the literature that sB7H3 values are increased in other malign tumors and some autoinflammatory diseases (type 1 DM, Sjogren’s syndrome, mycoplasma pneumonia, etc.) [19–21]. Therefore, we cannot say that sB7H3 has a high specificity in the diagnosis of BC. It is important to exclude accompanying inflammatory diseases in future studies examining the relationship between sB7H3 and BC. Additionally, although the blood test is much more practical, B7H3 levels detected at the tissue level may provide more meaningful results. Finally, the low number of patients and the low density of triple-negative patient subgroups may be other reasons for the insignificant results we obtained concerning the relationship between clinicopathological variables, sTIL levels and sB7H3.
Conclusions
To the best of our knowledge, this study is the first to examine the relationship between sB7H3 and disease parameters in BC patients. We found that sB7H3 may be a clinically practical and meaningful biomarker in differentiating BC from BBD. In order to evaluate the relationship of B7H3 with clinical variables and especially with sTILs in BC, studies conducted with tumor tissues from larger numbers of patients are needed.








