Introduction
Since the time of performing the first pneumonectomy (PN) in 1933, the number of total lung resections constantly decreases. Increasingly PN is being replaced by more parenchyma-sparing techniques like sleeve-lobectomy (SL) [1–3].
Pneumonectomy is associated with both higher mortality and postoperative complication rates compared to SL [1]. Also, the indications for PN have been decreasing for several years [2]. Despite the lower number of PNs, which has been observed in recent years, it is still necessary for 15–20% of patients with non-small cell lung cancer (NSCLC) to undergo total lung resection due to local malignancy of the disease [3]. Pneumonectomy is generally indicated for malignant tumours, infections or degenerative lesions, that cannot be treated with more parenchyma-sparing resections [4]. Despite the larger perioperative trauma and higher mortality rate [5], both elements – operative technique and the postoperative care – have improved and favourable outcomes of 5-year survival and quality of life (QoL) are achievable [3]. Very important for each patient who is going to undergo PN is the expected QoL and possible consequences of the operation [4–8]. The higher operation quality and improved postoperative care lead to higher expectations regarding the daily activity after the operation [9]. The role of the doctor should be not only choosing the most effective and radical method of treatment but also explaining to the patient the consequences of that choice, engaging the patient in the treatment process. That is why patients should be obligatorily informed about the impact of the total lung resection on the QoL in the preoperative period [10].
Aim
The aim of the study was to assess the type and the frequency of postoperative complications, the long-term results, and the QoL after PN in NSCLC patients.
Material and methods
In the total group of 1160 patients, operated on in 2008–2011 due to NSCLC, 192 patients underwent PN (16.6%). There were 44 women and 148 men in the analysed group, in the age range of 32–79 years (mean: 60). Diagnostic imaging (CT scan, PET/CT), endoscopy (bronchofibroscopy, autofluorescence bronchoscopy, EBUS-TBNA), and the other examination procedures (“blind” transbronchial biopsy, transthoracic needle aspiration biopsy) were performed during the diagnostic process and qualification for PN. The patients with locally operative tumours (evaluation based on CT scan and bronchofibroscopy), excluded from distant metastases (PET/CT, abdominal and brain CT, brain NMR and bone scintigraphy) and mediastinal lymph nodes metastases (PET/CT, EBUS, mediastinoscopy), with favourable results of the studies assessing efficiency of respiratory and cardiovascular systems were qualified for PN. In the functional assessment of the respiratory system spirometry, diffusing capacity of the lung for carbon monoxide (DLCO) and capillary blood gas screening were routinely performed. In some patients also a 6-minute walk test and stair test were carried out. In doubtful situations, the predicted postoperative value of the forced expiratory volume in 1 second (ppoFEV1) and predicted postoperative diffusing capacity of the lung for carbon monoxide (ppoDLCO) were calculated. Cardiovascular evaluations were assumed according to electrocardiography (ECG) and echocardiography. Some cases required additional studies (exercise test, Holter monitoring, coronary angiography). Pneumonectomy was performed under general intravenous anaesthesia with bronchial intubation and anterolateral thoracotomy access. In 13% of cases, the lung hilum vessels were supplied intrapericardially. In 150 (78.1%) patients the bronchial stump was closed with a manual suture (double layer of the continuous PDS 3-0 suture), in the remaining 42 (21.9%) with the mechanical suture (linear stapler). The bronchial stump was strengthened with tissue flaps (intercostal muscle, pericardium, thymus, pleura or mediastinal fat tissue) in 162 (84.4%) patients. Each patient underwent complete mediastinal lymphadenectomy.
Obtaining the postoperative histopathological examination result was followed by assessing the stage of the cancer according to the 7th edition of the 2009 TNM classification. Each patient was consulted by an oncologist and the qualification for adjuvant therapy (chemo- and/or radiotherapy) depended on the malignancy of the cancer, the general condition and the presence of accompanying diseases.
Clinical data were obtained using thoracic surgery and oncology departments’ medical records. The patients’ postoperative follow-up was based on the information from thoracic surgery and oncology clinics and also the questionnaires that were sent to the patients.
Statistical analysis
Statistical calculations were performed using Statistica 12.0 PL software (StatSoft Polska, Kraków, Poland) or StatXact 9.0 (Cytel Inc., Cambridge, MA, USA). Categorical data were analysed with the χ2 test or the Fisher-Freeman-Halton test. All results were considered significant at p < 0.05. Analysed data are presented as means and standard deviations, medians and interquartile ranges or absolute numbers and/or percentages, as appropriate. The unpaired t-test was used to analyse the data with normal distribution and homogeneous variances. Normality of the distribution was tested with the Shapiro-Wilk test, and the equality of variances was checked with Levene’s test. The data that did not follow a Gaussian distribution were analysed with the Mann-Whitney U test. The relationship between variables was analysed with Spearman’s rank correlation coefficient and by multiple logistic regression. For overall survival we used the Kaplan-Meier method in a univariate analysis, and differences between subgroups were assessed by the log-rank test.
The QoL was analysed using version 3.0 of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) and the EORTC QLQ-LC13 module. The results have been calculated and compared with the EORTC’s reference values for the general population and for all cancer patients [11]. After receiving the permission from EORTC to use the questionnaires, they were sent to the patients in April 2015 and collected from May to July 2015. The EORTC QLQ-C30 is a standardized and complex instrument for examining the symptoms of cancer or the patient’s general condition and functioning during treatment. The EORTC QLQ-C30 consists of questions that are divided into three scales: the Symptoms Scale, the Global Health Status (QoL) and the Functional Scale. The possible degrees of assessment for the items were “not at all”, “a little”, “quite a bit”, and “very much” with the exception of the two questions that evaluate the Global QoL ranging from (1) “very poor” to (7) “excellent”. Cronbach’s α of the Global QoL scale was 0.94. A high score on the Symptom Scale (Cronbach’s α = 0.83) represents a high level of symptomatology. A high Global Health Status (Cronbach’s α = 0.94) and Functional Scale (Cronbach’s α = 0.89) represent a high QoL and high level of functioning. The questionnaire also includes a specific LC13 supplement, which includes 13 questions and incorporates one multi-item scale to assess typical lung cancer symptoms such as coughing, dyspnoea, haemoptysis and pain in the chest [11]. The questionnaire response rate was 72%.
Results
Among 192 operated patients in 83 right PN and in 109 left PN were performed. The most common histological type of the tumour was squamous cell carcinoma (71.4%). Adenocarcinoma was diagnosed in 20.3%. For 139 (72.4%) patients the most common comorbidities were: hypertension (33.9%), chronic obstructive pulmonary disease (23.4%), ischaemic heart disease (12%), diabetes (9.9%), history of myocardial infarction (8.9%) and arrhythmia (4.7%). 16.1% of patients were operated on in stage I, 47.4% in stage II, and 35.4% in stage III. Metastases in lymph nodes N1 were observed in the group of 87 (45.3%) patients and in 49 (25.5%) the N2 stage was confirmed. 52.7% of patients were treated with chemo-and/or radiotherapy. Thirteen patients underwent preoperative chemotherapy with a satisfactory response to treatment. Table I shows the clinical and pathological data in the analysed group of patients. The mortality rate during hospitalization was 4.2% and was higher after right-sided PN (4.8% vs. 3.7%). A number of factors that could potentially have influence the postoperative mortality rate (age, gender, TNM staging, preoperative FEV1, preoperative FVC, operated side, resection of additional structures, intrapericardial resection, bronchopleural fistula, postoperative bleeding, other complications, blood transfusion, comorbidities, preoperative chemotherapy) were statistically analysed. The only factor that significantly affected the mortality rate was a history of myocardial infarction. Postoperative mortality in this group was 17.6% and among the other patients 2.9% (p = 0.02).
Table I
Study group patients’ clinical characteristics
The time of the hospitalization was 5–98 days (mean: 17). The time spent in the Intensive Care Unit was 0–66 days (mean: 6).
Postoperative complications occurred in 109 (56.7%) patients. The most common were as follows: atrial fibrillation (31.8%), anaemia requiring transfusion (29.7%), renal failure (19.3%), bleeding requiring reoperation (6.8%), psychosis (5.7%). In 8.9% of cases, bronchopleural fistula was found, more often on the right side (14.5% vs. 4.8%, p < 0.05). The occurrence of bronchopleural fistula or other complications did not significantly affect postoperative mortality or long-term survival. Table II shows the rate of selected postoperative complications.
Table II
Postoperative complications frequency
The percentage of 5-year survival in the entire population was 44.8%. Overall 5-year survival rates for individual TNM stages of cancer were: I – 62%, II – 50%, III – 33% (p < 0.05). 63.3% of N2 patients, 35.6% of patients with N1 and 26.8% of N0 patients died in the observation period (p < 0.05). The factors that significantly influenced the long-term results in univariate analysis were: pT stage, pN stage, pTNM stage, intrapericardial resection, resection of extrapulmonary structures and postoperative chemotherapy. In multivariate analysis, four features significantly affected the 5-year survival: pTNM stage (Fig. 1), pN stage (Fig. 2), intrapericardial resection and resection of additional extrapulmonary structures (Table III).
Table III
Survival univariate and multivariate analysis
Fig. 1
Overall survival curves according to pTNM stage: Kaplan-Meier accumulated survival ratio reveals significant difference (p < 0.05) between time survived rate in patients with pTNM I, II or III (p = 0.017)
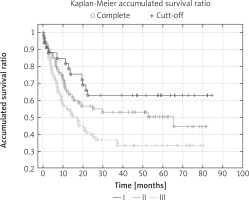
Fig. 2
Overall survival curves according to pN stage: Kaplan-Meier accumulated survival ratio reveals significant difference (p < 0.05) between time survived rate in patients with NO/N1 or N2 (p = 0.013)
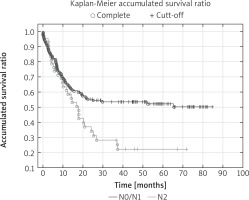
The risk of death after additional resection was more than 6 times higher compared with those without resection of extrapulmonary structures (OR = 6.4). The intrapericardial resection increased the risk of death twofold (OR = 2.8). The risk of death in the absence of adjuvant chemotherapy was more than 2 times higher compared to those who had adjuvant chemotherapy (OR = 2.5). There was a relationship between the N stage and the survival rate. The number of deaths in the N0/N1 group differed significantly (44.2%) from the number of deaths in the N2 group (68.2%).
The EORTC QLQ- LC13 questionnaire consisted of 43 questions, which patients were able to answer by choosing the numerical value to describe the intensity of a given object. The EORTC QLQ-LC13 questionnaire consisted of questions that were divided into four categories: Symptoms Scale, Functional Scale, Global QoL and the EORTC LC13 supplement, which is the 13-item lung cancer-specific questionnaire module. The results were compared to the EORTC QLQ-LC13 References Values. Only 3% of patients rated their general condition and QoL as “very bad”. Seventy-four percent rated this category from “medium” to “excellent” (4 to 7 points, 50,8). The pain rate was 32.8. Also, 22.6% of patients did not have any pain or the pain did not disturb their daily activity. The most common and intensive symptom that patients were suffering from was dyspnoea (48.1–54.3). Over 16% of patients rated these symptoms as “very often” (4 points). Many patients presented high cognitive functioning (76.3%). The physical functioning was presented as follows: 3–8% of patients have very often difficulties with a short walk or they have to rest in bed or on a couch during the day (4 points), 84% of patients do not need any help with eating, dressing, washing or using the toilet (1 point) (Table IV).
Table IV
Results of the EORTC QLQ-C30 and EORTC QLQ-L13 questionnaires
The questionnaire consists of questions that were divided into four categories: Symptoms Scale, Functional Scale, Global Quality of Life and the EORTC LC13 supplement, which is the 13-item lung cancer-specific questionnaire module. A high score for Symptom Scale represents a high level of symptomatology. A high Global Health Status and Functional Scale represent a high quality of life and high level of functioning. The LC13 supplement assesses the intensity of typical lung cancer symptoms.
Discussion
In the last few decades, the number of PNs performed in patients with NSCLC has been systematically decreasing and currently in most thoracic surgery departments does not exceed 15% of the total anatomical resections [1, 12]. The decreasing number of the PNs is associated with, on the one hand, the higher number of detected cases of early-stage lung cancer with low-dose CT scans, and on the other hand by the increasingly frequent performance of lung resection using parenchyma-sparing procedures such as SL. Indications for both PN and SL are centrally located tumours, invading the main bronchial or vascular structures of the hilum of the lung. Many authors report favourable early and long-term results of the SLs, highlighting the lower complications rate, the higher 5-year survival rate, and better QoL compared to PN [12, 13]. However, it is crucial that the SL can be performed only in a relatively small group of patients with favourable anatomical conditions. There is a fairly large population of patients with locally advanced lung cancer, whose only option for radical surgical treatment, and therefore a chance for complete recovery, is the PN. Although the postoperative mortality rate and the frequency of serious complications after resection of the entire lung are higher than after standard lobectomy or SL, the distant outcomes reported by some authors are encouraging. Riquet and co-authors analysed early and long-term results in a group of 1446 patients who underwent PN due to NSCLC. The postoperative mortality rate was 6.3%, complications occurred in 27% of patients and the 5- and the 10-year survival rates were 32% and 19% [14]. In other publications, the postoperative complication rate after PN ranges from 22% to 44% [15, 16]. The serious complication rate (life-threatening or requiring reoperation) is about 16% [1], the occurrence of bronchopleural fistulas ranges from 3.6% to 16% [15–17], and postoperative mortality is 3.6% to 11.2% [15, 18, 19]. Five-year survival rates in all TNM stages fluctuate between 21% and 39% [20, 21]. In the group analysed in this study complications occurred in 56.7% of patients, the postoperative mortality rate was 4.2% and the 5-year survival rate reached 45%. Bronchopleural fistula occurred in 8.9% of patients and was more frequent on the right side. Similarly to other authors, the TNM staging, especially N-stage, and the extension of the surgery (extended PN) were the determinants of the long-term outcomes. Unlike other reports [14, 22], in our material postoperative complications (including bronchopleural fistula) did not affect postoperative mortality or distant survival. A significant influence of age, gender or comorbidities on the survival rate was not observed as well. An interesting observation was the relatively high (21%) 5-year survival rate in the group of patients with metastatic N2 disease. The N2 feature is certainly one of the most important unfavourable predictors. Although in Riquet’s publication 26% of patients with PN who survived 10 years had in the postoperative material metastases in N2 lymph nodes [14], these are significantly better outcomes than those seen in patients treated conservatively (chemoradiotherapy) [23]. Based on the results of recently published cohort studies [15, 17] and meta-analyses [12, 18], SL is recommended for patients with centrally located lung cancer as long as it is technically feasible.
Obviously, the PN should be still in the arsenal of surgical procedures performed in patients with NSCLC. Performing it in some clinical situations remains controversial, such as in patients with N2 disease, in patients after induction therapy or in patients with high operation risk [4]. Another problem is the QoL after such extensive surgery. For many patients, the risk of significant worsening of the QoL after surgery is an important argument in choosing the type of therapy. Some of them are willing to accept the higher risk of the complications in the postoperative period, but they would not accept the significant deterioration in the distant QoL [24, 25]. Although the results of QoL studies performed using the standardized questionnaires indicate a significant deterioration in the PN group [3], the QoL is not as dramatic as might be expected after such extensive resection. Some authors claim that QoL after PN is comparable or only slightly deviates from the QoL after the SL [1].
According to our questionnaire’s results, the QoL is favourable. Lots of symptoms normally associated with postoperative trauma or cancer grading are not observed after such a long (5–7 years) period after PN. Comparing PN with SL, Balduyck et al. observed significant differences in patients’ physical functioning, role functioning, social functioning, cognitive functioning, and shoulder dysfunction, but their study included only one year after the operation [24, 25]. Andersson et al. compared the survival rate and the general QoL in a long period of time (mean: 69 months) and described no statistically significant differences in both objects [1]. The problem of the QoL in patients 5–7 years after PN is not widely described in the literature. It requires research that will compare PN to the more parenchyma-sparing procedures (e.g. SL). Also, the evaluation of the results should be analysed in age-divided groups. Our results suggest that PN patients could lead life at a satisfactory level. Additionally, the survival rate (45%) allows us to claim that in properly qualified cases, there is still a place for PN as an effective method of treatment of NSCLC.
Conclusions
Our study confirmed a relatively high risk of postoperative complications in patients undergoing PN for NSCLC but it does not significantly increase perioperative mortality (4%) and long- term survival. Long-term results in this group of patients are satisfactory and the rate of 5-year survival reaches 45%. According to the questionnaire, the QoL is favourable and it is also higher than could be expected after such extensive resection.





