Introduction
Drought is considered a major abiotic stress and a serious agricultural concern that adversely affects plant seed yield by disrupting various physiological and biochemical processes. These processes include metabolite synthesis and assimilation, photosynthesis, respiration, ion transport, transpiration rate, and stomatal behavior (Bukhari et al., 2021; Saud et al., 2014).
While chickpea plants exhibit higher tolerance to drought compared to other cool-season legumes, water scarcity remains an important factor that reduces the productivity and economic yield of this plant (Karalija et al., 2022). The stress caused by water scarcity significantly increases the generation of reactive oxygen species (ROS) by interfering with electron transport chains and metabolite oxidation within plant cells. Superoxide radicals, hydroxyl radicals, and hydrogen peroxide (H2O2) can have a negative effect on protein structure, membrane lipid integrity, and nucleic acids (Soares et al., 2019). Plant survival under environmental stress conditions largely relies on the balance between ROS production and scavenging capacity. An imbalance in this process leads to oxidative stress, also known as secondary stress, in plants (Hasanuzzaman et al., 2020). Plants employ various mechanisms to cope with drought stress, including regulation of stomatal opening, osmotic adjustment, and enzymatic and nonenzymatic antioxidant systems. However, antioxidant enzymes play a crucial role in scavenging ROS and are essential for plant survival under stress conditions (Dvořák et al., 2021). Nonenzymatic antioxidants, such as carotenoid pigments, and antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), glutathione peroxidase (GPX), glutathione reductase (GR), glutathione S-transferases, and ascorbate peroxidase (APX), are key components of the plant’s antioxidant defense system (Rajput et al., 2021).
Following the green revolution, rapid population growth, urban migration, water resource reduction, and declining input efficiency have surpassed the progress made in agricultural production (Mahpul et al., 2021). Meeting future needs in agricultural production will require at least a 50% increase, necessitating revisions in management practices, the adoption of new technologies in agriculture, and the sustainable management and restoration of soil and water resources. Nanotechnology holds the potential in revolutionizing traditional fertilizer design and enhance food security (Mittal et al., 2020). Nanofertilizers, developed using nanoparticles, offer larger surface activity, increased surface reaction areas, and enhanced catalytic efficiency compared to conventional bulk particles (Hussain et al., 2023). Among the essential elements crucial for growth under environmental stress, zinc, and silicon have gained significant attention. Zinc is an essential trace element serving as a catalytic and structural cofactor in hundreds of enzymes, thus playing a vital role in plant growth and development (Umair Hassan et al., 2020). Zinc’s catalytic role in finger proteins (ZFP245 and C2H2 Zn) and enzymatic antioxidants (Zn-SOD) aids in the removal of ROS generated during drought stress (Han et al., 2020). The application of nano zinc under drought-stress conditions has been shown to significantly enhance antioxidant activities (Foroutan et al., 2019). Karami et al. (2016) observed increased activity of antioxidant enzymes, including superoxide dismutase, peroxidase, CAT, and proline, in soybeans following the foliar application of zinc under drought-stress conditions. Silicon, while nonessential for plant growth, has demonstrated beneficial effects when applied exogenously to various plant species (Liang et al., 2015; Spalevic et al., 2022). The application of silicon under drought stress conditions improves plant tolerance through multiple mechanisms, such as regulating aquaporin gene expression and reducing ROS, which inhibit aquaporin activity (Wang et al., 2021). Silicon enhances osmotic pressure under drought stress by promoting the accumulation of soluble sugars or amino acids in the xylem (Sonobe et al., 2010). It also improves root hydraulic conductivity by influencing root growth, increasing the root-to-aerial parts ratio, and enhancing aquaporin activity (RIOS et al., 2017). Improved root hydraulic conductivity facilitates water absorption, maintains photosynthetic rates, and enhances overall plant performance under water-deficient conditions (Chen et al., 2018).
While studies highlight the significant role of mineral nutrition in mitigating drought stress in plants, the simultaneous application of silicon and zinc, particularly in nanoforms, remains relatively unexplored. Thus, this study aims to evaluate the effects of foliar spray of silicon, zinc, and zinc-containing mesoporous silica nanoparticles (MSNPs-Zn) on the enzymatic ROS scavenging system in chickpea plants under water-stress conditions.
Materials and methods
Site and soil properties
To investigate the impact of zinc, silicon, and Zn-containing MSNPs on the Kabuli chickpea (Cicer arietinum cv. Azad), field trials were conducted at the Research Farm of the University of Maragheh, located in the northwest of Iran. The field’s coordinates were 46°16ʹ east longitude and 37°23ʹ north latitude, with an altitude of 1485 m above sea level. Based on Koppen’s classification, this region experiences a semiarid and cold temperate climate. The plants received a total of 142.2 mm of rainfall during the growing season, spanning from March to June.
Physicochemical analysis of the upper 40 cm of soil indicated that it was of clay loamy composition, with a pH of 7.25 and an EC of 1.06 dS/m. The soil contained 0.87% organic carbon, with available N at 0.03%, K at 360 mg/kg, P at 4.46 mg/kg, Fe at 3.12 mg/kg, and Zn at 0.14 mg/kg. Monthly rainfall, average temperature, and relative humidity data for the growing season are presented in Table 1.
Table 1
Temperature and precipitation in the field experiment area during the growing season of chickpea (2018)
Sample collection and experimental procedure
The field experiment was conducted during the 2018 growing season using a split-plot design based on a randomized complete block design with three replications. The main plots were assigned to different irrigation treatments, namely: irrigation at 90% of field capacity (FC) to maintain well-watered conditions, irrigation at 60% FC to induce moderate water stress, and irrigation at 30% FC to impose severe stress. The drought treatment began by withholding water from the flowering stage (R1: start of flowering with flower buds visible at any node on the main stem) until the harvest maturity stage (RH: 90% of pods have turned golden-brown). Before flowering and during the vegetative growth stages, all plots were adequately watered. The available soil water was measured using time-domain reflectometry with the TRIME-FM model from England. The amount of water applied for each stress level was calculated using the method described by Mahmoud et al. (2018).
The subplots were assigned to different foliar fertilizer treatments, including a control group without fertilizer, zinc sulfate application, silicon application, zinc sulfate + silicon combined application, and mesoporous nanoparticles of zinc and silicon. All treatments were replicated three times and followed a randomized scheme. The nanoparticles of silicon and zinc were obtained from Pishgaman Iranian Nanomaterial Company (Iran) and characterized using Transmission Electron Microscopy. The mesoporous zinc and silicon nanoparticles were synthesized following the method described by Shen et al. (2018). Foliar spraying was performed at three stages: flowering, podding, and seed filling, using a concentration of 100 ppm.
Before planting, the seeds were disinfected with benomyl fungicide (0.2% w/v). The plants were sown in March and harvested in July 2021. Seedbed preparation included a reversible moldboard plow at a depth of 15–20 cm in October 2020, followed by secondary tillage using a disc harrow at a depth of 10–15 cm, and planking 3 days before seeding. Each experimental unit had an area of 4 m2 (2 × 2) with a 2.0 m wide nonirrigated zone. The experimental unit consisted of eight rows, with each row measuring 2 m in length and spaced at 0.25 m intervals. The seeding density was 10 seeds per linear meter, resulting in a final population of 400 000 plants per hectare. Within each plot, a randomly selected quadrat measuring 50 × 50 cm in the central part of the plot was used for harvesting plant leaves 99 days after planting (R8: the beginning of seed formation in pods). Manual weeding was performed as needed throughout the crop’s development. Drip irrigation, with a frequency of 3 days, was employed until the flowering stage. Irrigation was then suspended, and subsequent irrigations were scheduled based on the specified percentages of the field capacity.
Extraction and measurement of biochemical traits
The young and fully developed upper leaves of the plants were collected at the beginning of the seed formation stage and immediately frozen in liquid nitrogen. They were then stored in a −80°C freezer for future use.
To extract the enzymes SOD, CAT, and GPX, as well as ascorbate, 0.5 g of leaf sample were homogenized in a mortar with liquid nitrogen. Subsequently, 5 ml of phosphate buffer (pH = 7) was added to the homogenized material. The samples were centrifuged for 15 min at 15 000 × g at 4°C (Sairam et al., 1998). For the extraction of the APX enzyme, the same method was used along with 5% polyvinyl pyrrolidine and ascorbate.
For the measurement of SOD enzyme activity, a reaction mixture consisting of sodium carbonate, methionine, EDTA, potassium phosphate buffer, distilled water, and the extracted enzyme was prepared (Sairam et al., 2002). The reaction was initiated by adding riboflavin, and the absorption of the samples was measured using a spectrophotometer (UV-1800, Shimadzu, Japan) at 560 nm (Sairam et al., 1998).
Activity of CAT enzyme was also measured according to the method described by Aebi (1984). The reaction mixture included KH2PO4 buffer, H2O2, ddH2O, and the enzyme solution. Enzyme activity was calculated at 290 nm using an extinction coefficient of 36.16 mmol/cm.
Guaiacol peroxidase (GPX) activity was determined by measuring the oxidation of guaiacol. The assay mixture contained 10 mmol/l potassium phosphate (pH 6.4), 8 mmol/l guaiacol, and 2.75 mmol/l H2O2.
To measure H2O2 concentration, 5 ml of trichloroacetic acid solution was added to homogenized leaf samples, followed by centrifugation at 1200 × g for 15 min. The resulting supernatant, along with a reaction complex containing phosphate buffer and sodium iodide, was read at 390 nm (Chen et al., 2000).
MDA content in the leaves was estimated following the method described by Heath and Packer (1968). Proline concentrations were determined according to the method described by Bates et al. (1973).
Carotenoids and chlorophyll concentrations were determined according to a method by Hiscox and Israelstam (1979). Leaf samples were homogenized in 50 ml 80% (v/v) acetone and centrifuged at 10 000 × g for 10 min. The absorbance of each acetone extract was measured at 666, 653, and 470 nm. Chlorophyll concentrations were estimated according to blow equations: Chl a = 15.65 A666 − 7.340 A653. Chl b = 27.05 A653 − 11.21 A666. Total carotenoid content was estimated through the above equation Car = 1000 A470 − 2.860 Chl a − 129.2 Chl b/245.
Statistical analysis
The collected data were analyzed using the appropriate analysis of variance (ANOVA) in SAS 9.1 statistical package (SAS Institute Inc., Cary). Principal component analysis was conducted using SPSS (IBM Corp., Armonk, NY, USA). To compare the means of different treatments, the least significant differences (LSD) test was performed at a significance level of 5%.
Results
The ANOVA for APX activity revealed significant effects of both water stress level and foliar spray treatments (Table 2). APX activity increased by 15% under moderate water stress levels (60% FC) compared to the well-watered condition. While all foliar treatments increased APX activity, the most effective results were observed under moderate stress conditions. Plants sprayed with nanosilicon particles, a combination of zinc and silicon, and MSNPs-Zn exhibited the highest APX activity. The foliar spray of MSNPs-Zn under severe water stress increased APX activity by 44% compared to untreated plants.
Table 2
Effect of foliar spray of silicon, Silicon dioxide nanoparticles and zinc-containing mesoporous silica nanoparticles on physiochemical parameters in chickpea plants under different drought stress levels
| APX | CAT | Cu/ZnSOD | MnSOD | FeSOD | H2O2 | MDA | Chl a | Chl b | Cart | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 90% FC | F1 | 8.09 hi | 0.244 g | 0.307 gh | 0.256 abc | 0.232 bcd | 2.81 bc | 30.13 cdef | 3.55 bcd | 1.19 def | 8.44 defg |
| F2 | 9.46 gh | 0.42 defg | 0.748 bc | 0.283 abc | 0.258 bc | 1.92 bc | 26.73 ef | 4.34 ab | 1.34 cd | 9.24 cde | |
| F3 | 11.03 bcde | 0.431 defg | 0.542 cdef | 0.306 ab | 0.270 bc | 1.95 bc | 28.06 def | 4.49 ab | 1.43 bc | 10.49 bc | |
| F4 | 11.39 bcd | 0.699 bcd | 0.613 bcde | 0.320 ab | 0.272 bc | 1.73 c | 26.68 ef | 5.11 a | 1.62 ab | 11.27 ab | |
| F5 | 12.11 b | 0.565 cdef | 0.656 bcd | 0.353 ab | 0.258 bc | 1.78 c | 25.55 f | 5.47 a | 1.77 a | 12.62 a | |
| 60% FC | F1 | 10.45 cdefg | 0.474 cdefg | 0.492 defg | 0.230 bcd | 0.232 bc | 5.24 a | 44.48 b | 2.42 def | 1.11 ef | 6.57 ij |
| F2 | 11.78 bc | 0.529 cdefg | 0.816 ab | 0.270 abc | 0.256 bc | 3.48 b | 35.14 bc | 2.90 def | 1.25 cde | 8.04 efgh | |
| F3 | 14.35 a | 0.765 bc | 0.745 bc | 0.216 abc | 0.261 bc | 3.20 bc | 34.03 cde | 2.95 cde | 1.27 cd | 7.63 ghi | |
| F4 | 14.31 a | 0.99 ab | 0.802 ab | 0.370 ab | 0.301 abc | 2.68 bc | 27.25 def | 3.52 bcd | 1.26 cde | 8.41 defg | |
| F5 | 14.97 a | 1.08 a | 0.969 a | 0.373 a | 0.378 a | 2.46 bc | 26.19 ef | 4.23 ab | 1.44 bc | 9.43 cd | |
| 30% FC | F1 | 7.76 i | 0.237 g | 0.203 h | 0.06 e | 0.105 e | 6.62 a | 52.80 a | 1.59 f | 0.84 g | 5.33 j |
| F2 | 10.06 defg | 0.349 fg | 0.431 efg | 0.110 de | 0.139 de | 3.40 bc | 37.41 bc | 1.95 ef | 1.01 fg | 6.76 hi | |
| F3 | 9.77 efg | 0.375 efg | 0.381 fgh | 0.010 de | 0.140 de | 2.60 bc | 37.02 bc | 1.90 ef | 0.97 fg | 6.90 hi | |
| F4 | 9.54 fgh | 0.687 bcde | 0.442 defg | 0.146 cde | 0.213 cd | 2.27 bc | 33.06 cdef | 2.34 def | 1.06 ef | 7.74 fghi | |
| F5 | 11.19 bcde | 0.78 abc | 0.538 cdef | 0.163 cde | 0.317 ab | 1.73 c | 30.414 cdef | 3.39 bcd | 1.23 cde | 9.09 def | |
| Statistical significance | |||||||||||
| S | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| F | ** | ** | ** | ** | ** | ** | ** | ** | * | * | |
| S×F | * | ** | NS | NS | NS | * | * | NS | * | ||
APX – ascorbate peroxidase (Units mg protein−1 min−1), CAT – catalase activity (Units mg protein−1 min−1), Cu/Zn-SOD – activity of copper/zinc superoxide dismutase isoezyme, Mn-SOD – activity of manganese superoxide dismutase, Fe-SOD – activity of iron superoxide dismutase, H2O2 – hydrogen peroxide concentration (mg/g FW), MDA – malondialdehyde content (nmol/g FW), Chl
F1 – foliar spray of distilled water as control (check), F2 – zinc sulfate F3 – SiO2 nanoparticles, F4 – zinc sulfate+silicon, F5 – foliar spray of mesoporous nanoparticles of zinc and silicon; in each column rows with different letters have statistically significant differences at the 5% level (P ≤ 0.05);
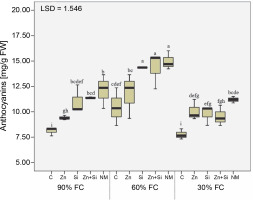
Evaluation of anthocyanin content in upper leaves indicated that severe water stress decreased the concentration of this pigment by 8% compared to the well-watered condition. The highest anthocyanin concentration was observed in plants grown under moderate water stress conditions and sprayed with Si, Zn + Si, and MSNPs -Zn (Fig. 2). Although foliar spraying of MSNPs-Zn had more positive effects on anthocyanin levels across all irrigation levels compared to other foliar treatments, its effectiveness was more evident at 60% FC. The lowest amount of anthocyanin was observed in plants without foliar spraying under favorable moisture conditions and severe drought stress.
Fig. 1
Transmission electron microscopy (TEM) images of mesoporous silica nanoparticles (A) and zinc oxide (B) nanoparticles
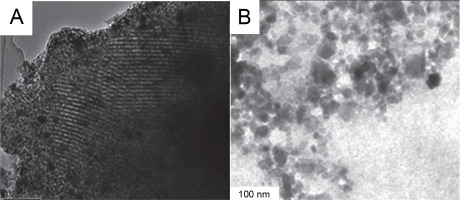
Fig. 2
The effects of foliar application of nutrients on anthocyanin content in chickpea plants grown under different water stress levels; three irrigation levels (irrigation to 90, 60, and 30% field capacity; FC) was applied starting from flowering stage; C – foliar spray of distilled water as control (check), Zn – zinc sulfate, Si – SiO2 nanoparticles, Zn + Si – zinc sulfate + silicon, NM – foliar spray of mesoporous nanoparticles of zinc and silicon; different uppercase letters in columns indicate a statistical difference according to LSD test (P < 0.05)
Assessment of CAT activity showed that moderate water stress increased its activity by 68% compared to the control (well-watered condition). However, in plants grown under severe stress conditions, CAT activity did not differ significantly from plants grown under 90% FC (Table 2). Foliar feeding at all irrigation levels increased CAT activity, with the highest effect observed under moderate water stress through the application of Zn + Si and MSNPs-Zn.
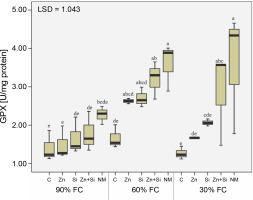
Evaluation of GPX showed that moderate and severe stress-induced its activity by 60 and 32%, respectively, compared to the well-watered condition. Although the foliar application of nutrients increased GPX activity by an average of 70% compared to the control, the highest enzyme activity was recorded in plants treated with foliar application of MSNPs-Zn (Fig. 3).
Fig. 3
Activity of guaiacol peroxidase in chickpea plants affected by foliar feeding of Zn and Si and different water stress levels (90% FC, 60% FC and 30% FC – irrigation to 90% FC, 60% FC and 30% FC, respectively); C – foliar spray of distilled water as control (check), Zn – zinc sulfate, Si – SiO2 nanoparticles, Zn + Si – zinc sulfate + silicon, and NM – foliar spray of mesoporous nanoparticles of zinc and silicon
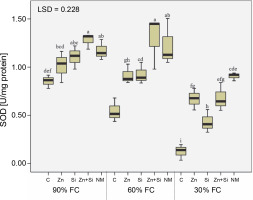
Investigation of SOD isozymes (Cu/Zn-SOD, Mn-SOD, and Fe-SOD) revealed that severe water stress decreased their activities by 40, 62, and 31%, respectively. However, among the isozymes, only Cu/Zn-SOD isozyme was stimulated under moderate water stress conditions, with its activity increasing by 33% compared to the control condition (Table 2). The behavior of total SOD activity was similar to Mn-SOD and Fe-SOD, with its activity reduced by 51% under severe stress compared to 90% FC (Fig. 4). While all foliar sprays increased the activity of SOD isozymes, the greatest effect was observed with Zn + Si and MSNPs-Zn under moderate stress conditions.
Fig. 4
The effects of foliar application of nutrients on activity of superoxide dismutase in chickpea plants grown under different water stress level; three irrigation levels (irrigation to 90, 60, and 30% field capacity; FC) was applied from flowering stage; C – foliar spray of distilled water as control (check), Zn – zinc sulphate, Si – SiO2 nanoparticles, Zn + Si – zinc sulfate + silicon, NM – foliar spray of mesoporous nanoparticles of zinc and silicon
Examination of H2O2 concentration indicated that moderate and severe stress increased the concentration of this active oxygen species by 67 and 63%, respectively, compared to the control (Table 2). However, foliar spray of zinc and silicon in all forms decreased H2O2 concentration, and the highest concentration (6.62 mg/g FW) was recorded in plants grown under severe stress with foliar spray of distilled water (control). A similar trend was observed for malondialdehyde (MDA).
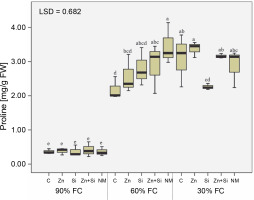
Assessment of proline concentration showed that regardless of stress intensity, drought stress caused a seven-fold increase in free proline content in the upper leaves (Fig. 5). Interestingly, foliar spraying did not have a significant effect on proline content under well-watered conditions. However, foliar spraying of zinc-containing compounds (bulk or nano) significantly increased free proline in the leaves, with the highest amount recorded in plants treated with foliar application of MSNPs-Zn under moderate stress.
Fig. 5
The concentration of proline in chickpea plants affected by foliar feeding of Zn and Si and different water stress levels (90% FC, 60% FC, and 30% FC – irrigation to 90% FC, 60% FC and 30% FC, respectively); C – foliar spray of distilled water as control (check), Zn – zinc sulphate, Si – SiO2 nanoparticles, Zn + Si – zinc sulfate + silicon, NM – foliar spray of mesoorous nanoparticles of zinc and silicon
The evaluation of photosynthetic pigments revealed that foliar feeding of nutrients significantly increased the concentrations of Chl a and Chl b under all moisture regimes. However, the positive effects of foliar feeding were more prominent on Chl a compared to other photosynthetic pigments (Table 2). Mean comparison analysis showed that both traditional bulk zinc and zinc in the nanostructure form significantly improved the levels of photosynthetic pigments at all irrigation levels, with a greater effect observed under severe drought stress conditions.
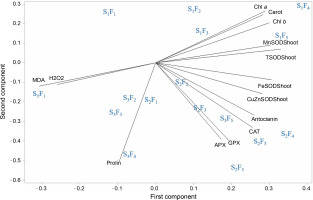
The biplot in Figure 6 provides a vector view of the traits and combined treatments, illustrating the interrelationship among all measured traits and the superiority of the combined treatments. A low angle and positive correlation were observed between APX, GPX, CAT activities, and anthocyanin concentration. Additionally, a positive correlation was recorded among total SOD, Mn-SOD, Chl a, Chl b, and carotenoids (r = cos 0° = +1). The highest values of these traits were observed in plants sprayed with Zn + Si and MSNPs-Zn under well-irrigated conditions (S1F4, S1F5). Interestingly, there was no correlation between free proline concentration and CAT, anthocyanin, and Cu/Zn-SOD, as indicated by a 90° angle (r = cos 90° = 0). Furthermore, a significant negative correlation was observed between MDA or H2O2 and SOD isozymes, photosynthetic pigments, and carotenoid concentration (r = cos 180° = −1). The combined treatments closest to MDA or H2O2 were distilled water spray under severe stress conditions (F1S3). The combined treatments closest to APX, CAT, GPX, and anthocyanin were the foliar spraying of various nano and bulk forms of zinc under moderate water stress conditions (S2F3, S2F4, S2F5).
Fig. 6
Biplot of principal component analysis (PCA); eigenvalues of the correlation matrix symbolized as vectors representing the traits measured in the study and spatial distribution of different multimodal treatments; low angles between lines show a high positive correlation; APX – ascorbate peroxidase, CAT – catalase activity, Cu/Zn-SOD – activity of copper/zinc superoxide dismutase isoezyme, Mn-SOD – activity of manganese superoxide dismutase, Fe-SOD – activity of iron superoxide dismutase, H2O2 – hydrogen peroxide concentration, MDA – malondialdehyde content, Chl a – chlorophyll a concentrations, Chl b – chlorophyll b concentration, Cart – carotenoids concentration; S1, S2, and S3 represent different water stress levels and indicate the irrigation to 90% field capacity, 60% field capacity and 30% field capacity, respectively; F1 – foliar spray of distilled water as control (check), F2 – zinc sulphate, F3 – SiO2 nanoparticles, F4 – zinc sulfate + silicon, F5 – foliar spray of mesoporous nanoparticles of zinc and silicon
The cluster analysis of the combined treatments resulted in the formation of three clusters (Fig. 7).
Fig. 7
Cluster analysis of combined treatments (water stress level and foliar feeding) in terms of similarities in influencing the activity of antioxidant enzymes and biochemical characteristics of chickpeas under field conditions; S1, S2, and S3 represent different water stress levels and indicate the irrigation to 90% field capacity, 60% field capacity and 30% field capacity, respectively; F1 – foliar spray of distilled water as control (check), F2 – zinc sulphate, F3 – SiO2 nanoparticles, F4 – zinc sulfate + silicon, F5 – foliar spray of mesoporous nanoparticles of zinc and silicon
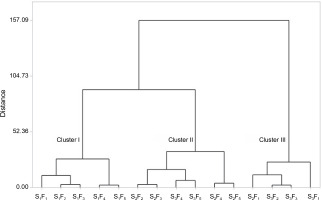
Cluster I comprised different foliar sprays under well-watered conditions. This cluster did not exhibit significant improvements in the investigated traits, except for photosynthetic pigments.
Cluster II included the most effective treatments. This group consisted of foliar sprays of different forms of zinc under moderate and severe stress (S2F3, S2F4, S2F5, S3F3, S3F4, and S3F5), as well as a foliar spray of silicon nanoparticles under moderate water stress. These treatments demonstrated the highest stimulating effects on defense systems, including antioxidant enzymes, the accumulation of compatible solutes, and protective pigments.
Cluster III comprised the least effective combined treatments, which often involved spraying distilled water under medium and severe stress conditions (Fig. 7).
Discussion
Global climate change over the past decades has intensified water scarcity issues, leading to widespread drought conditions on a global scale. This situation is particularly severe in semiarid regions and poses the greatest threat to food security. Our study results demonstrate that water stress significantly affects various biochemical parameters, resulting in reduced concentrations of key components such as photosynthetic pigments, carotenoids, and anthocyanins. The concentration of chlorophyll provides information about a plant’s photosynthetic potential and its ability to produce photo-assimilates. Consistent with our findings, other studies have also reported decreased chlorophyll and sun-screen pigment content in response to drought (Macar and Ekmekçi, 2008; Valifard et al., 2012; Khazaei et al., 2020). However, under moderate stress, increased anthocyanin content can be attributed to its protective roles. Anthocyanins act as filters, preventing excessive light absorption by leaves and minimizing photoinhibition. One possible explanation for this phenomenon is that anthocyanin accumulation under moderate drought stress confirms its potential role as an osmoregulator, maintaining water homeostasis, and acting as sunscreen and scavengers of ROS (Naing and Kim, 2021).
Moderate drought stress increases the activity of antioxidant enzymes. While low rates of ROS are produced as normal byproducts of cell metabolism, increased ROS production is a common feature of abiotic stress (Mansoor et al., 2022). This occurs due to disruption of intracellular redox balance, deviation of electron transfers, and the undesired transfer of electrons to nontarget molecules such as oxygen. The increase in H2O2 concentration levels during drought stress confirms this point. Stomatal closure and nonstomatal limitations (e.g., damage to photosynthetic enzymes and dysfunction of photosynthetic cycles) are among the main reasons for increased ROS generation during drought stress. However, plants are capable of managing disturbances and restoring the redox state to the desired homeostasis through adaptive and protective mechanisms (Santos et al., 2019). Increasing the expression of genes encoding ROS scavengers and enhancing the activity of antioxidant enzymes are important protective mechanisms (Postiglione and Muday, 2020). In our study, moderate drought stress significantly increased the activity of CAT, GPX, APX, and Cu/Zn SOD. Evaluation of three SOD isoenzymes, namely, Mn-SOD (mitochondria), Fe-SOD (chloroplasts), and Cu/Zn-SOD (cytosol, chloroplasts, and peroxisomes), revealed that Cu/Zn-SOD was more responsive to the applied treatments, which may be due to its pervasive and ubiquitous presence in plant cells.
Another important finding was that under severe drought stress, the total activity of SOD decreased compared to optimal moisture conditions. This decrease can be attributed to the detrimental effects of drought stress on the three-dimensional structure of enzymes, disruption of protein folding, reduced gene expression, and severe damage to intracellular structures (Yang et al., 2021). The application of zinc and silicon in both conventional bulk and nano forms improved the activity of these antioxidant enzymes under water deficit conditions. Their positive effect was more pronounced when applied together, particularly under moderate stress. Zinc and silicon are mineral nutrients that enhance water use efficiency, plant nutrition, accumulation of osmoregulators, expression and activity of antioxidants, and photosynthesis and protective pigments (Biju et al., 2017; Umair Hassan et al., 2020; Malik et al., 2021; Sattar et al., 2021). Zinc, as a cofactor of many key enzymes, regulates the biosynthesis of phytohormones such as auxin and abscisic acid. Silicon also plays a role as a beneficial element in stimulating defense and regulatory processes (Spalevic et al., 2022). Our findings are consistent with those of El-Zohri et al. (2021), who reported that foliar spraying of green-synthesized Zn-NPs induced SOD, CAT, and APX activities, and reduced drought-induced oxidative stress, as indicated by the decreased levels of MDA and H2O2 compared to untreated controls. Zn has been reported to exhibit a protective role against ROS-induced membrane damage (Sattar et al., 2021), which was confirmed by our results showing low levels of MDA in zinc-treated plants. Zn application can minimize drought-induced lipid peroxidation while maintaining plasma membrane stabilization (Umair Hassan et al., 2020).
The significant positive effects of zinc and silicon on the measured traits, even under well-watered conditions, may be attributed to the significant deficiencies of these elements in the soil of semiarid areas. Considering the unfavorable physicochemical conditions of the soil in these areas, foliar feeding can be suggested as one of the best supplemental methods. However, the best effects were observed with the combined application of Zn and Si in the form of MSNPs-Zn under moderate stress conditions. MSNPs-Zn, with their ability to retain more moisture and gradually release zinc and silicon nanoparticles over a longer period, facilitate rapid absorption of nanoparticles by the shoots and easier delivery of beneficial nanoparticles to target points inside plant cells. The presence of mesopores in MSNPs-Zn may explain their superiority over other sol-sprayed compounds. Factors such as stomatal closure reduced vascular flows, and disruption of physiological processes (Yang et al., 2021) might hinder the significant impact of nanoparticles under severe drought stress. However, further research is required to understand the gene expression and accumulation patterns of different antioxidants and osmo-regulators following the foliar application of nanoparticles.
Conclusion
The results of the performed experiment indicate that foliar spray of silicon and zinc significantly reduced membrane damage in plants. As the severity of drought stress increased, the levels of photosynthetic pigments, carotenoids, and anthocyanins decreased in the tested plants. However, foliar spraying of conventional zinc and silicon fertilizers, as well as MSNPs-Zn, was able to improve the levels of these pigments under drought-stress conditions. The foliar spraying treatments enhanced the antioxidant system, and the lowest amount of H2O2, which is an indicator of oxidative stress, was observed in plants sprayed with MSNPs-Zn.
The results of the experiment suggest that under moderate drought stress, foliar feeding of conventional Zn + Si fertilizer and MSNPs-Zn can mitigate the damage caused by drought stress. This effect is largely attributed to the stimulation of adaptive mechanisms such as increased accumulation of compatible solutes and enhanced scavenging of ROS. Considering the water scarcity in semiarid areas, especially during the planting of second-season crops and the irrigation limitations during the reproductive stage of chickpeas, foliar spray of MSNPs-Zn can be considered as a suitable agro-management option to alleviate the negative effects of drought stress.










