Introduction
Breast cancer is the most common cancer among Singaporean women [1]. Radiotherapy (RT) has been indicated in at least 50% of all breast cancer patients during the initial treatment, of whom at least 60% of all breast cancer patients have an indication for RT at some point in the course of illness [2, 3]. Adjuvant breast cancer RT has benefits for local recurrence rates and long-term survival, whilst whole-breast irradiation reduces the local recurrence rate by 70–88% [4, 5] with a 5.3% reduction in overall mortality after 15 years. However, there are concerns about radiation-induced heart disease (RIHD), particularly in left-sided breast cancers and in those who require regional nodal irradiation. To date, several large cohort studies have reported on the impact of radiation on cardiac outcomes in terms of ischaemic cardiac events such as coronary artery stenosis, myocardial infarction, and cardiac death [6, 7].
Darby et al. reported that the risk of major acute coronary events (ACE) increased linearly with the mean dose to the heart by 7.4% per gray [6]. A meta-analysis of breast cancer patients who received left-sided RT were found to have increased risks of developing cardiac disease, cardiac death, and death from any cause, as compared to those who received right-sided RT [6, 7]. Compared to breast cancer patients without RT, patients with RT had higher risks of coronary heart disease- and ischaemic heart disease (IHD)-related mortality. However, these trials predominantly used older RT techniques, resulting in considerable doses to the heart [6, 7].
Since then, there have been major advancement in RT techniques, such as three-dimensional (3D) treatment planning, cardiac shielding, prone position, and deep inspiration breath hold, which have led to a continuous reduction in radiation doses to the heart. There are also limited studies describing the long-term effects of these modern RT techniques and on potential interactions of radiation with other risk factors for cardiovascular diseases (CVD). Taylor et al. analysed mean heart doses (MHD) from left tangential RT to cardiac structures over decades, and they described reductions in MHD from 13.3 Gy in the 1970s, to 4.7 Gy in the 1990s, and 2.3 Gy in 2006 [8]. This decrease seemed to have resulted in a very low risk of death caused by RIHD, at least for women without CVD [9]. A Surveillance, Epidemiology, and End Results (SEER) registry analysis of 48,353 women with breast cancer over 65 years old also concluded that RT did not increase the risk of acute myocardial infarction in more than 10 years [10].
Ischaemic heart disease remains in the top 3 causes of death in Singaporean females, accounting for 15.7% of deaths in 2019 [11]. Few data are published on the IHD risk factors in an Asian cohort of irradiated breast-cancer patients. Chang et al. in an analysis of 2577 women from a Korean breast cancer registry, who underwent breast conservation and adjuvant RT from 1990–2012, did not find an excess risk in ACE between left-sided and right-sided breast-cancer patients [12].
Material and methods
The primary endpoint of the study is to investigate the IHD-related mortality and overall mortality between left-sided and right-sided breast-cancer patients.
This is a registry-based, single centre, retrospective cohort study. Eligible breast cancer patients were diagnosed and treated between January 2000 and December 2016 at the National Cancer Centre Singapore (NCCS). We included all non-metastatic patients with histologically confirmed breast cancer with either an invasive carcinoma or a carcinoma in situ, who had undergone definitive treatment with curative intent. Exclusion criteria included previous or synchronous cancer history, bilateral breast cancers, and palliative treatments.
Individual data on breast cancer disease, therapy, and comorbidities were extracted from the Joint Breast Cancer Registry (JBCR). The following data fields were extracted: date of diagnosis, age at diagnosis, date of birth, height/weight, laterality, comorbidities, TNM-stage (tumour, node, metastasis), histology subtype, and hormonal profile (oestrogen, progesterone, HER2-expression). Treatment information such as surgery, chemotherapy regime, adjuvant endocrine therapy, and RT treatment information were also extracted. Data on the first and subsequent recurrences were obtained.
Baseline IHD risk factors, defined as any personal history of IHD, diabetes (DM), hypertension (HYPT), hyperlipidaemia (HLD), chronic renal failure, and cerebrovascular disease, were obtained from JBCR, the Singapore Cardiac Data Bank (SCDB) registry, and/or from the electronic records system for administering RT. The SCDB was established in 2000 as a National Data Bank of CVD and procedures. It is a comprehensive source for Singapore National data containing information of over 80% of hospital care delivered in public institutions.
The baseline date was defined as the date of diagnosis. Patient event times were censored in cases where a new radiation treatment was delivered in the follow-up period, in cases of death, or at the end of follow-up time. The follow-up interval was defined as the time between baseline and censoring date or date of event. Patient information was collected until the last known date of review. The underlying causes of death were coded according to the 10th revision of the International Classification of Diseases. The Registry of Births and Deaths records information of all deaths of Singapore residents. All deaths certified related to IHD were analysed. These included ischaemic cardiomyopathy and ischaemic heart failure. All-cause mortality, breast cancer-specific mortality, and all other causes of death were analysed. Individual follow-up started with the date of diagnosis of primary breast cancer. The end of the follow-up was defined as the date of death, last information date, or 31 December 2020, whichever occurred first.
In NCCS, breast irradiation techniques have evolved during the study period. For patients up to 2009, RT was performed using 2 tangential fields and a single anterior field for SCF nodes. From 2010 onwards, 3D conformal RT using computed tomography (CT)-based planning was used with field-in-field optimization. Beam configuration comprised tangential fields and additional anterior and/or posterior beams for nodal irradiation. Doses to the heart, lungs, and contralateral breast were minimized.
Standard conventional fractionation of 50 Gy in 25 fractions was prescribed for the target volume, with a sequential boost of 10–16 Gy in 2 Gy per fraction depending on pathologic risk factors. The whole heart was contoured according to established guidelines. START hypofractionation, 40 Gy in 15 fractions, were standard dose prescription from 2012 onwards [13]. In 2014, intensity modulated radiotherapy (IMRT) delivered in the form of helical tomotherapy was prescribed for advanced breast cancer patients with 4 or more positive nodes (pN2), who required internal mammary chain (IMC) nodal irradiation. There were 251 tomotherapy treatments from 2014 to 2016, which is a very small subset of RT patients.
The Ethics Committee approved the use of the database for analysis.
Statistical analysis
We used descriptive statistics to characterize patient demographics, stages of breast cancer, patterns of treatment, and adjuvant systemic therapy for the whole cohort. Categorical variables were summarized as frequency and percentage, and continuous variables were summarized using mean, standard deviation, median, interquartile range (IQR), and range. Median follow-up was estimated using the reverse Kaplan-Meier method. Univariable and multivariable Cox regression analyses were performed to assess the association between overall survival and IHD-related mortality with clinicopathological and treatment characteristics. A two-sided p-value less than 0.05 was considered statistically significant. We adjusted for potential confounders, which included the following: baseline IHD risk factors, application of chemotherapy, hormonal therapy, stage of cancer, and age at diagnosis. Overall survival was defined as the time interval between initial diagnosis and death by any cause or the last follow-up date. Ischaemic heart disease-related mortality was defined as the time between initial diagnosis and death from IHD or last follow-up date. All statistical analyses were carried out using R software (version 3.6.3).
Results
Description of cohort
A total of 14,419 non-metastatic breast-cancer patients were included in the analysis. All the patients were diagnosed after 2000, and 35.6% of patients were diagnosed after 2011. The median follow-up time was 8.6 (5.1–13.0) years. The median age was 52 (IQR 45–60) years. Most (70.5%) of the histology subtypes were luminal A or B. Only 8.6% patients had HER2-enriched breast cancer because HER2 receptor testing was routinely done only in the later cohort. 63.2% were diagnosed with early stage I and II breast cancers. Those who did not receive RT largely consisted of patients with either ductal carcinoma in situ (DCIS) or early-stage node-negative breast cancer patients who underwent mastectomy (88.4%), and thus did not require adjuvant RT. Hence, the irradiated group had a larger proportion of advance stage of disease, thus needing chemotherapy and RT.
66.3% (9556/14419) of the cohort had adjuvant RT, and the distribution in year of diagnosis, age, race, histology, staging, the application of systemic therapy, and the type of surgery were similar for left- and right-sided tumours (Table 1). A history of baseline IHD risk factors was confirmed in approximately 30% of the patients. For reference, the population prevalence in Singapore for HYPT, DM, and HLD is reported to be 21.5%, 8.6%, and 33%, respectively [14].
Table 1
Patient characteristics including baseline ischaemic heart disease risk factors
[i] CVD – cerebrovascular disease, CRF – chronic renal failure, DCIS – ductal carcinoma in situ, DM – diabetes mellitus, ER – oestrogen receptor, HER2 – human epidermal growth factor receptor 2, HLD – hyperlipidaemia, HYPT – hypertension, IHD – ischaemic heart disease, IQR – interquartile range, LCIS – lobular carcinoma in situ, PR – progesterone receptor, SD – standard deviation, TIA – transient ischaemic attacks, TNM – tumour, node, metastasis Patients are considered to have positive IHD risk factor, if they have any one of the pre-existing comorbidities (IHD, DM, HYPT, HLD, cerebrovascular disease, CRF).
Radiotherapy treatment dosimetry
The radiotherapy treatment details are described in Table 2. Patients were classified as belonging to a CT-based RT planning period from 1 Jan 2010 onwards. The available dosimetry in MHD was recorded. The MHD ranged 0–21.5 Gy for right-sided RT and 0–18.8 Gy for left-sided RT. The average MHD was 0.9 Gy for right-sided RT and 2.6 Gy for left-sided RT. Hypofractionation and standard fractionations of RT was delivered to similar proportions of left and right-sided RT patients.
Table 2
Radiotherapy details
Mortality data
At the end of the follow-up period, more than 82% of the cohort patients were still alive, with 70% of them without any disease recurrence (Table 3). A total of 61 patients (0.4%) were lost to follow-up, and 2593 patients were reported to be deceased at the time of analysis. In the non-RT group, the proportion of deaths was 15.8%, compared to 19.1% in the irradiated group. The rest of the vital statuses of the cohort can be seen in Table 3.
Table 3
Vital status of cohort
Table 4 summarises the cause of death for all patients in this study. Notably, IHD accounted for 6% of the total deaths. In the non-RT group, IHD accounted for 9.5% of all known causes of death. In the RT group, of those with right-sided tumours, 4.2% died of heart disease, and in left-sided breast cancer, heart disease represented 4.9% of all causes of death. There were a small number of patients (n = 10) in whom both IHD and breast cancer were listed as major contributing causes of death. There were more breast cancer-related deaths in the RT group (75.3% /71.8%) vs. 54.6% in the non-RT group. The non-RT group had a higher proportion of DCIS patients and early-stage invasive breast cancer (44% vs. 25.3%/24.7%). The RT group had a higher proportion of stage 3 patients (28% vs. 5.2%).
Table 4
Description of deaths by cause of death according to radiotherapy group and laterality
Overall, for breast cancer patients receiving RT, our results showed similar mortality rates between left-sided and right-sided cancer. Comparison of MHD from the CT-based period showed a difference of 1.7 Gy between RT of the left- and right-sided breast cancer (Table 2). This small difference may not be large enough to cause a rise in the mortality.
To adjust for confounders on IHD-related mortality, a multivariate model analysis was done, which included age at diagnosis, use of chemotherapy, and pre-existing IHD, as covariates (Fig. 1). In the irradiated group, our results showed no significant difference for laterality of breast cancer irradiation (Fig. 1). The hazard ratio (HR) of IHD-related mortality for left-sided versus right-sided RT was 0.94 (95% CI: 0.64–1.38). A history of pre-existing IHD significantly increased the IHD-related mortality risk (HR 4.18, 95% CI: 2.41–7.25) in the RT group, while the HR was 5.34 (1.94–14.70) in the non-RT group. The use of chemotherapy was not associated with an increased risk (HR 1.12, 95% CI: 0.70–1.79); see Supplementary Table 1. However, other IHD risk factors such as HYPT, DM, and HLD did not show an expected positive relationship when included in the model. This is probably due to a large number of missing data for these comorbidities.
Fig. 1
Hazard ratios for ischaemic heart disease-related mortality stratified for breast cancer with and without radiotherapy
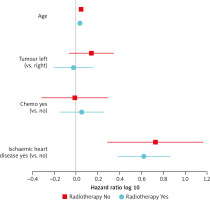
For comparison, we also assessed the effect of laterality on cardiac mortality in the group without RT in Figure 1. The hazard ratio for left-sided versus right-sided breast cancer was not statistically significant, at 1.38 (95%CI: 0.87–2.21).
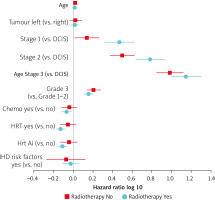
Another multivariate model analysis was done for death from all causes with breast-cancer stage, the use of chemotherapy, hormonal therapy, and baseline IHD risk factors as variables (Fig. 2). For patients treated with RT, left- vs. right-sided breast cancer patients did not reveal any significant differences in all-cause mortality with HR 1.03 (95% CI: 0.94–1.13). As expected, the HR for overall mortality increased with increasing stages of diseases. Chemotherapy and hormonal therapy exerted a protective effect for overall mortality with an HR of 0.85 (95% CI: 0.75–0.95, p = 0.005) and 0.74 (95% CI: 0.66–0.83, p < 0.001), respectively, for patients who received RT (see Supplementary Table 2). For the non-RT group, the HR for patients who received chemotherapy or hormonal therapy was not statistically significant, but it trended towards a lower HR.
Fig. 2
Hazard ratios of all-cause mortality stratified by with or without radiotherapy. The known prognostic factors are age, laterality, stage (compared against ductal carcinoma in situ), grade, receipt of chemotherapy, receipt of hormonal therapy, receipt of hormonal therapy with aromatase inhibitors, baseline induced heart-disease risk factors
Hrt Ai – hormonal therapy with aromatase inhibitors, IHD – induced heart disease
Discussion
In this study, we present the mortality outcomes of a large cohort of Asian breast-cancer patients treated in a single institution from 2000 onwards with a median follow-up of 8.7 years. We did not find a significant increase in IHD-related mortality between left-sided and right-sided RT cohorts, with HR of 1.08 and 1.38, respectively. Multivariate analysis did not show any effect of laterality on IHD-related or all-cause mortality. We noted a higher proportion of IHD-related deaths in the non-RT group (9.5%) versus the irradiated group (4.5%). This is probably because breast cancer is a competing cause of death in this group of women, given the higher proportion of more advanced breast-cancer disease. The RT group is more likely to die from breast cancer earlier rather than IHD or other causes, thus removing a subject from being at risk for IHD-related death in our study.
Our study concurs with the findings of recent studies on the risks of IHD-related toxicities. Most studies that reported increased ACE included patients who received RT before the 1970s and primarily included patients treated with older two-dimensional RT techniques. Advancements in RT through the use of 3D planning, heart sparing, and IMRT techniques have resulted in comparable cardiac outcomes between left- and right-sided RT in recent studies due to reductions in cardiac doses [8, 15].
A large recent Danish cohort showed that there was no increased risk of ACE within the first 10 years after RT when CT-based simulation and planning were used. However, there was a higher risk of ACE in the left-sided vs. right-sided breast cancer patients treated earlier in the non-CT based period [16]. Another large cohort study based on the SEER cancer registry by Henson et al. in 2013 [17] demonstrated that while breast-cancer patients treated with RT in an older era (1973–1982) showed increased risk of cardiac mortality, patients treated in the modern era (after 1993) using CT planning did not show any differing RT-related cardiac mortality with regard to laterality [17]. A large study in Germany with 11,982 breast-cancer patients treated between 1998 and 2008 also showed that contemporary RT is not associated with an increased risk of IHD-related mortality with regard to laterality [18]. See Table 5 for a summary of these studies.
Table 5
Recent cohort studies on cardiac mortality in breast-cancer patients related to radiation therapy
| Year, authors | Country | Cohort size, cohort age information | Registered diagnosis year | Follow-up information | Outcome | Results of left-sided vs. right-sided (95% CI) |
|---|---|---|---|---|---|---|
| 2005, Darby et al. [19] | USA | 308,861, ages 20–79 | 1973–2001 | Until 1st Jan 2002, death, loss to follow-up, or 85th birthday | Cardiac mortality | Registered diagnosis 1973–1982 < 5 years: RR = 1.19 (0.98–1.45) 5–9 years: 1.21 (0.97–1.50) 10–14 years: RR = 1.42 (1.11–1.82) ≥ 15 years: RR = 1.58 (1.29–1.95) Registered diagnosis 1983–1992 < 5 years: RR = 1.00 (0.84–1.20) 5–9 years: 1.08 (0.90–1.29) ≥ 10 years: 1.27 (0.99–1.63) Registered diagnosis 1993–2001 < 5 years: RR = 0.95 (0.79–1.14) 5–9 years: RR = 0.99 (0.73–1.35) |
| 2010, Bouchardy et al. [20] | Switzerland | 1245, mean age 57.4 years | 1980–2004 | Until 30th Dec 2006, mean follow-up of 7.7 years | Cardiovascular mortality | HR (adjusted) = 0.52 (0.24–1.12) |
| 2011, McGale et al. [21] | Denmark, Sweden | 72,134, age < 80 years | 1976–2006 | Until 31 Dec 2006, death, heart disease diagnosis, loss to follow-up, or 90th birthday | Mortality from heart disease | All ischaemic heart disease RR = 1.00 (0.86–1.15) Heart disease other than ischaemic heart disease RR = 1.00 (0.81–1.22) |
| 2013, Henson et al. [17] | USA | 558,871, ages 20–79 | 1973–2008 | Until 1st Jan 2009, death, loss to follow-up, or 85th birthday | Cardiac mortality | Registered diagnosis 1973–1982 < 10 years: 1.19 (1.03–1.38) 10–14 years: 1.35 (1.05–1.73) 15–19 years: 1.64 (1.26–2.14) ≥ 20 years: 1.90 (1.52–2.37) Registered diagnosis 1983–1992 < 10 years: 0.99 (0.87–1.12) 10–14 years: 1.02 (0.83–1.24) 15–19 years: 1.11 (0.86–1.43) ≥ 20 years: 1.21 (0.72–2.04) Registered diagnosis 1993–2002 < 10 years: 0.97 (0.89–1.06) 10–19 years: 0.90 (0.71–1.15) Registered diagnosis 2003–2008 < 10 years: 1.00 (0.82–1.23) |
| 2014, Rutter et al. [22] | USA | 344,831, median age 59.7 years | 1998–2006 | Median follow-up 6.04 years (0–14.17 years) | Overall survival | DCIS HR = 0.995 (0.925–1.069) Invasive breast cancer with breast RT only HR = 0.983 (0.962–1.004) Invasive breast cancer with breast and regional nodes RT HR = 0.868 (0.682–1.126) (Sensitivity analyses restricted to patients with at least 10 years of follow-up) |
| 2016, Boero et al. [23] | USA | 72,134, ages 66–80 | 2000–2009 | Until December 2010, or death | Cardiac mortality | HR = 1.08 (0.96–1.21) |
| 2017, Merzenich et al. [18] | Germany | 11,982, mean age 64.0 years | 1998–2008 | Until December 2012, or death. Median follow-up 6.3 years | Cardiac mortality | HR = 0.94 (0.64–1.38) |
| 2021, Milo et al. [16] | Denmark | 29,662, age range not provided | 1999–2016 | Median follow-up 7.9 years | Cardiac events (coronary artery disease and valvular heart disease) | Registered diagnosis 1999–2007 < 5 years: 1.33 (0.80–2.24) 5–10 years: 1.16 (0.70–1.96) ≥ 10 years: 1.95 (1.12–3.53) Registered diagnosis 2008–2016 < 5 years: 0.92 (0.66–1.26) 5–10 years: 0.91 (0.57–1.43) ≥ 10 years: 0.62 (0.05–5.40) |
There could be several reasons that contributed to our findings. The patients in our study were treated in an era where breast cancer screening, systemic treatment, and RT techniques have progressed. Before 2009, RT techniques used in NCCS were a pair of tangential fields with a combined anterior field for supraclavicular nodes. Computed tomography-based simulation and planning was introduced thereafter in our institution, when 3D conformal technique combined with cardiac shielding and an optional boost became the standard of care. Regional nodal irradiation including the IMC chain were only routinely prescribed for patients with pN2 disease from 2015 onwards, which comprised of only 2.6% of the irradiated patients. Our institution had tight cardiac dose constraints and utilized cardiac shielding even before the introduction of CT-based simulation and planning. In 2007, the awareness of RIHD in left-sided BC patients increased [24]. Our heart-dose constraints were based on the principle of ALARA, but we aimed to keep the MHD below 4 Gy for left-sided RT. After the publication of a report by Darby et al. [6], we reduced our target MHD to < 2 Gy. This was reflected in our low MHD for left-sided breast cancers at 2.6 Gy in the CT-based cohort (2010–2016). In our study, we also did not detect an increase in IHD-related mortality with respect to laterality. With a MHD dose difference of only 1.7 Gy between the left- and right-sided cohorts, it may be too small to detect a significant difference in IHD-related mortality rates.
A strength of this study is that it provides IHD-related mortality data on Asian breast cancer women. To our knowledge, our study is one of the few studies that included Asian breast-cancer patients receiving RT, which may differ from a Western cohort of patients. Our study showed low rates of smoking (3.5%) and obesity (11.3%), which is similar to a Korean cohort study of breast cancer [12] in which there were 3.4% of smokers and only 3% of patients with body mass index (BMI) > 30. This is in contrast with other non-Asian cohorts with a median BMI of 28 and obesity rates as high as 44% [27].
Darby et al. reported that the dose-dependent risk increase in ACE started less than 5 years after RT and continued more than 20 years later [6]. The median follow-up time in our study was 8.66 years, which is comparable to most registry studies. However, the CT cohort median follow-up time is shorter, at 5.7 years, which is a limitation of our study. An extended period of follow-up is required to further investigate late IHD events, because our number of IHD-related mortality events in the irradiated group is small, at n = 83. Ischaemic heart disease accounted for 6% of total deaths, which is smaller than expected, for several reasons. One reason is that the study cohort consisted of younger women (median age 52 years), and the age-specific mortality rate of IHD goes up markedly from 70 years old onwards [14]. Another possible reason is that the mortality data of non-residents who have left the country are not captured in our database, but there is little impact on final results because they form a small proportion (< 4%). Other limitations intrinsic to retrospective studies apply to our study.
We have limited data of the patients’ pre-existing IHD risk factors because they were not systematically collected in the registry. There is a significant amount of missing information with regards to pre-existing baseline IHD risk factors, as reflected in Table 1. Our cohort prevalence of DM, HYPT, and HLD is 40–50%, which is significantly higher than the national prevalence rates of 8.6–33% [14]. One possible reason is that patients without any comorbidities tend to be under-reported in the database, which was extracted from clinical records, and they were categorized as ‘unknown’. Thus, the true number of patients without any IHD risk factors was likely to be much larger than our study results show. The true prevalence of IHD risk factors in our cohort is likely to be on par with the national prevalence rates. Given the large size of our cohort, with comparable unknowns in the IHD risk factors in both groups, this limitation should have little or no impact on our final study results.
As per studies of the general population, IHD risk factors have been linked to future cardiovascular events in survivors receiving cardiac radiation across various cancer types [28]. Particularly in breast cancer patients undergoing RT, it has been shown that the presence of these risk factors doubles the risk of an ACE, and pre-existing IHD increases this risk six-fold [28]. For our patients with pre-existing IHD, the risk of IHD-related mortality was increased in both the irradiated group and non-RT group.
Future study directions include a longer follow-up in the CT-based cohort and a precise dose assessment to characterize radiation doses in the cardiac substructures. There is also increasing evidence to show that doses in the coronary arteries and left ventricle are important determinants of RIHD [29, 30], and the predictive value of the MHD is not good for cardiac substructures [31]. Current treatment guidelines recommend treating IMC nodes for high-risk node-positive patients, resulting in MHD reaching > 6 Gy for left-sided treatment [32] even with IMRT. Hence, these patients may have increased risks for RIHD, similar to the cohort of patients treated in the 1970s–1990s.
Conclusions
Our study of Asian breast cancer patients did not reveal a significant increase in the risk of IHD-related mortality or overall mortality comparing left- and right-sided breast cancers in the modern era of RT. However, laterality is a crude measure for doses to cardiac substructures, and future efforts are needed to determine the dose-response relationship of patients’ risk of RIHD strike off as RIHD already means radiation-induced heart disease.








