Introduction
Depression is a multifaceted disorder with diverse causes, associated with the risk of severe medical illnesses. Many studies have shown that depression is mediated by pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α, interleukin (IL)-6 and IL-1 [1, 2]. Attenuation of peripheral inflammation is indicated as one of the possible mechanisms of action of antidepressants which are used for treatment of the major depressive disorder. Antidepressants block the reuptake of serotonin and/or norepinephrine into the presynaptic nerve terminals, resulting in enhanced synaptic monoamine levels. Many studies have shown that antidepressants from several classes, decrease production of pro-inflammatory cytokines [3, 4].
Inflammation is a biological response with multiple factors which act in a complex network. The entrance of leukocytes including neutrophils and macrophages into the site of inflammation is critical for the inflammatory process. Macrophages are important inflammatory cells involved in the initiation of inflammatory responses, and play critical roles in the development of acute inflammation by secreting various pro-inflammatory mediators including TNF-α, IL-6, cyclooxygenase-2 (COX2), and inducible nitric oxide synthase (iNOS) [5].
Nitric oxide (NO), which is produced by inducible iNOS, acts as a regulatory molecule with homoeostatic activities. However, it can be pathogenic when it is produced excessively [6]. Prostaglandin E2 (PGE2), which is synthesized from arachidonic acid by COX2, also plays a major role as a mediator of the inflammatory responses [7]. Inhibition of NO and PGE2 by blocking the expression of iNOS and COX2 in macrophages is a strategy in the development of anti-inflammatory agents. Lipopolysaccharide (LPS) is a potent activator of macrophages which produce inflammatory mediators such as iNOS and COX2 [8]. Many researchers reported that the inflammatory effect induced by another potent activator, carrageenan, could be also associated with an increase in NO and prostaglandin levels [9].
Maprotiline is a tetracyclic antidepressant possessing similar effectiveness to other antidepressants in treating mental depression [10]. Maprotiline mainly acts as a selective norepinephrine reuptake inhibitor (SNRI) with some effects on histaminergic, adrenergic and cholinergic receptors [11-13].
In previous studies, we and others demonstrated that maprotiline exerts a considerable anti-inflammatory activity on carrageenan-evoked paw edema in rats [14, 15]. Therefore, in this study, we aimed to further examine the possible mechanisms involved in the anti-inflammatory activity of maprotiline. In our previous works, we reported that maprotiline inhibited the release of pro-inflammatory cytokines such as IL-1β and TNF-α and decreased the migration of polymorphonuclear (PMN) leukocytes into the site of inflammation [16].
This study attempts to further investigate the possible molecular mechanisms involved in anti-inflammatory properties of maprotiline. Therefore, we evaluated the effect of maprotiline on the expression of iNOS and COX2 using an in vitro model of LPS stimulated human U937 macrophage cells as well as in vivo model of carrageenan-induced paw edema in rats.
Material and methods
Chemicals
Human monocytic cells (U937) were purchased from Pasteur Institute (Tehran, Iran). RPMI 1640 cell culture medium, fetal bovine serum (FBS), trypsin-EDTA and [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) were obtained from Gibco (USA). Phorbol myristate acetate (PMA), LPS from Escherichia coli 055:B5, dimethyl sulfoxide (DMSO) and dexamethasone were obtained from Sigma-Aldrich (USA). Maprotiline was donated by Iran Daru Pharmaceutical Co. (Tehran, Iran) and was dissolved in phosphate buffer saline (PBS) for cells and in isotonic saline for rats. Carrageenan (lambda) was purchased from Fluka Chemical (Switzerland) and was dissolved in isotonic saline.
Human U937 macrophages cell culture
The human monocyte cell line U937 was grown in complete RPMI 1640 medium supplemented with 10% (v/v) FBS at 37°C in a humidified atmosphere of 95% air and 5% CO2. Antibiotics, penicillin (100 U/ml) and streptomycin (100 μg/ml) were added to the cell culture during the growth phase, but removed prior to experimental manipulation. To differentiate the cells into adherent macrophages, they were seeded at a density of 5 × 105 cells/well and incubated for 48 hours in the presence of PMA at the final concentration of 100 nM into the cellular medium. The cells were then washed and incubated in normal growth medium for additional 24 hours prior to the addition of LPS (1 μg/ml). Different concentration of maprotiline from 10-8 M to 10-6 M was added to the medium one hour before addition of LPS (1 μg/ml). Cells with LPS alone and control cells (without LPS and component) also were included. Cells were used for assessment of cell viability by MTT assay and for the measurement of mRNA levels of COX2 and iNOS by real-time PCR.
Cell viability assay
To evaluate the toxicity of maprotiline and LPS on monocyte-derived macrophages, MTT assay was utilized. The ability of the cells to convert MTT shows mitochondrial activity and in consequence cell viability [17]. In this assay, cells were seeded in 96-well plates at a concentration of 1 × 104 cells/well. Cells were incubated with the concentration of LPS (1 μg/ml) and three different concentrations of maprotiline (10-8 M, 10-7 M and 10-6 M) for 24 h at 37°C. After incubation, the medium was removed and replaced with 100 μl RPMI 1640 phenol red free. Then 10 μl of (12 mM) MTT stoke was added to each well. The cells were incubated for 4 h at 37°C. Finally the MTT crystals were dissolved by adding 50 μl of DMSO solution and the formazan blue dye was read in a microplate reader (BioTek Instruments, Epoch, USA) at 570 nm.
Animals
Male Wistar rats (200-250 g) were obtained from the animal house of the Faculty of Pharmacy, Isfahan University of Medical Sciences, Iran. Animals were housed in standard polypropylene cages, four per cage, under a 12 : 12 h light/dark cycle with free access to food and water. The experiments were carried out in accordance with local guidelines for the care of laboratory animals of the Isfahan University of Medical Sciences.
Carrageenan-induced paw edema
Rats received a subplantar injection of 100 μl of a 1% (w/v) suspension of carrageenan lambda in the right hind paw [18]. The volume of the paw was measured by Plethysmometer (Ugo Basile, Italy) immediately before and then, 4 h after the carrageenan injection. Data were expressed as the increase in paw volume (ml) and compared with control values.
Experimental design
Doses which applied in the present study were 25 and 50 mg/kg and were chosen according to our previous report [16]. In the first series of tests, effect of i.p. maprotiline on carrageenan-induced paw edema was studied. Four groups (n = 6 rats in each group) were used in this study. Maprotiline was given intraperitoneally 30 min before subplantar injection of carrageenan. The control group received only vehicle. A group of animals were pretreated with dexamethasone (1 mg/kg, i.p.) and served as the positive control. Paw volumes (ml) were determined prior to carrageenan injection, and at the end of the experiment (4 h later). Then animals were scarified and the inflamed paw tissues (30 mg) were snap frozen in liquid nitrogen and stored at −80oC until they were used for RT-PCR analyses.
In the second series of tests, to confirm our previous study [16], the effect of i.p. maprotiline was evaluated on the PMN leukocytes infiltration in the inflamed paw tissue. Briefly, animals were divided into four groups (six rats in each group), including vehicle group (subplantar injection of carrageenan), i.p. maprotiline (25 and 50 mg/kg) and dexamethasone (1 mg/kg, i.p.) groups. Four hours after the carrageenan injection, animals were euthanized, and the carrageenan-treated paws were removed for pathological assessment.
Histopathologic examination
From carrageenan-treated paws in the second part, three samples were removed and fixed by immersion in 10% formaldehyde solution for several days. Then the fixed tissues were embedded in paraffin and cut into 3-4 μm slices. The slices were mounted on the glass slides and stained with hematoxylin and eosin for light microscopy analysis. The assessment was led by a pathologist in a blinded way.
Real-time PCR
Real-time PCR was performed for the detection of the mRNA expressions of COX2 and iNOS. Total RNA was isolated from U937 macrophages and homogenized paw tissues by Gene Jet RNA purification kit (Thermo Scientific, [EU] Lithuania) according to the manufacturer’s instructions. The concentration and quality of RNA preparations were determined by a spectrophotometer (BioTek Instruments, Epoch, USA) and gel electrophoresis. Standardized amounts of RNA were reverse-transcribed to cDNA using RevertAid first strand cDNA synthesis kit (Thermo Scientific, [EU] Lithuania) according to the manufacturer’s protocol.
The primers sequence for COX2, iNOS and housekeeping gene 18srRNA for human cell lines and rat were designed from the sequence list of GenBank database (National Centre for Biotechnology Information – NCBI) by using Beacon designer 8 software and then blasted against GenBank database sequences. Primer sequences are shown in Table 1.
Table 1
Primer sequences of interested genes in humans and rats
Real-time PCR was performed using SYBRGreen (Thermo Scientific, [EU] Lithuania) detection in Corbett machine, Rotorgene 6000 (Australia). Master Mix in each reaction tube includes cDNA, H2O, SYBR Green, forward and reverse primer of genes of interest.
The cycling conditions were as follows: initial denaturation at 95°C for 3 minutes and amplification for 45 cycles (95°C for 12 seconds for the denaturation, 60°C for 45 seconds for the annealing and extension). The relative amount of gene expression, normalized to the internal control 18srRNA. Validation of the reference gene (18srRNA) and the amplification efficiencies of targets and reference were performed [19]. The fold-change for each sample was analyzed by the 2-ΔΔCT method. The 2-ΔΔCT values obtained from these analyses directly reflect the relative mRNA quantities for the specific gene in response to a particular treatment [20]. Amplification of specific transcripts was further confirmed by obtaining melting curve profiles. All samples were run in triplicate.
Statistical analysis
The data are expressed as means ± S.E.M. One way ANOVA (analysis of variance) was performed to define the significance of treatment. To determine the specificity of treatment, Tukey post-hoc test was used to define differences between control and treatment groups. Differences were considered as significant for p < 0.05. Statistical analysis was performed using the SPSS 19 software.
Results
Cell viability
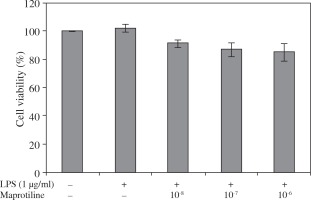
To evaluate if maprotiline and LPS in concentrations used in the experiments are toxic on U937 macrophages, cells were treated with increasing concentrations of maprotiline (10-8 M to 10-6 M) and LPS (1 μg/ml) for 24 h and cell viability was measured using MTT assay. Data obtained from U937 macrophages illustrated that LPS alone and in combination with different concentrations of maprotiline from 10-8 M to 10-6 M, had no significant effect on cell viability in comparison to untreated cells (Fig. 1).
Inhibition of LPS induced mRNA expression of COX2 and iNOS by maprotiline in U937 macrophages
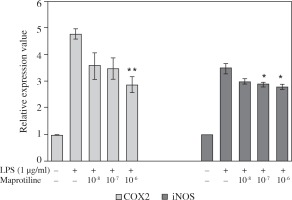
We also evaluated the effect of maprotiline on the expression of COX2 and iNOS in U937 macrophages stimulated with LPS. As demonstrated in Figure 2, maprotiline significantly decreased COX2 expression at a concentration of 10-6 M, whereas the expression of iNOS was considerably reduced both at 10-6 M and 10-7 M.
Fig. 2
Effect of maprotiline on LPS-induced U937 macrophages expression of iNOS and COX2. Cells pretreated with the indicated concentrations of maprotiline for 1 h, and then cells were activated with LPS (1 μg/ml). After 6 h, RNA was purified from cells and transcription of COX2 and iNOS was determined by quantitative RT-PCR. The mRNA expression data were normalized to the 18srRNA signal. Fold changes relative to control are presented. Mean ± SEM values of experiments are shown. *p < 0.05, **p < 0.01, compared with LPS alone treated group
Effect of intraperitoneal injection of maprotiline on carrageenan-induced paw edema
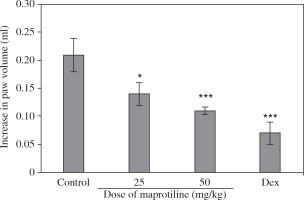
To evaluate the in vivo anti-inflammatory effect of maprotiline, a carrageenan-induced paw edema test was performed. As shown in Figure 3, i.p. injection of maprotiline at doses of 25 and 50 mg/kg noticeably inhibited the development of paw edema after the induction of inflammation as compared to the control group. As expected, the reference drug, dexamethasone (1 mg/kg), also caused a significant inhibition of post-carrageenan edema (Fig. 3).
Fig. 3
Effect of IP administration of maprotiline on carrageenan-induced paw edema in the rat. Maprotiline or the vehicle was administrated 30 min prior to carrageenan (1%) injection and rats were evaluated for paw edema at 4 h post-carrageenan. A group of animals received dexamethasone (1 mg/kg, i.p.) and served as a reference group. The values represent the mean changes in the paw volume ± SD (n = 6, *p < 0.05, ***p < 0.001 compared with the control group). Dex – dexamethasone
Histological examination
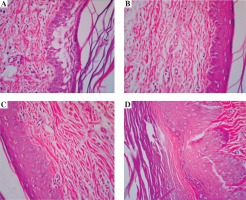
As shown in Figure 4, cellular infiltration and edema were obvious in the paw biopsies of control animals who just received carrageenan. Tissue PMN infiltration was reduced by i.p. maprotiline (Fig. 4B, C). At a dose of 50 mg/kg, maprotiline clearly decreased migration of PMN and edema but at 25 mg/kg, the effect of maprotiline was limited. The reference drug, dexamethasone (1 mg/kg), also exhibited a significant inhibitory effect on PMN infiltration and edema (Fig. 4D).
Fig. 4
Histopathologic examination of paw tissue of rats treated with maprotiline, 4 h after subplantar injection of carrageenan. A) Carrageenan-injected paw tissue in the vehicle-treated group. Migration of leukocytes mainly neutrophils and vasodilatation with edema were detected. B) Carrageenan-injected paw tissue of rats treated with maprotiline (25 mg/kg, i.p.). The migration of PMN leukocytes was reduced in a limited way. C) Carrageenan-injected paw tissue of rats treated with maprotiline (50 mg/kg, i.p.). The infiltration of PMN and edema was noticeably reduced. D) Carrageenan-injected paw tissue of rats treated with dexamethasone (1 mg/kg, i.p.). The PMN infiltration and edema was reduced considerably as compared with the vehicle-treated group. Sections were stained with hematoxylin and eosin, magnification ×40
The inhibitory effect of maprotiline on the expression of COX2 and iNOS in carrageenan-induced paw edema in rats
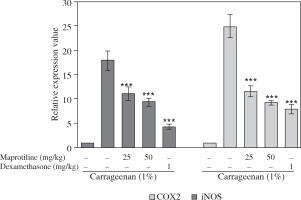
As illustrated in Figure 5, maprotiline at doses of 25 mg/kg and 50 mg/kg significantly decreased the expression of COX2 in inflamed tissue. Similarly these doses reduced the expression of iNOS. As expected, the reference drug, dexamethasone (1 mg/kg), showed a significant iNOS and COX2 mRNA reduction in carrageenan-induced paw edema.
Fig. 5
Effect of maprotiline on the gene expression of iNOS, and COX2 in carrageenaninduced paw edema in rats. Maprotiline at doses of 25 and 50 mg/kg was given 30 min before subplantar injection of carrageenan (1%). The control group received only vehicle. A group of animals that was pretreated with dexamethasone (1 mg/kg) was used as the positive control. RNA was purified from inflamed frozen paw and transcription of iNOS and COX2 was determined by quantitative RT-PCR. The mRNA expression data normalized to the 18srRNA signal. Fold changes relative to controls are presented. Mean ± SEM values of experiments are shown. ***p < 0.001 compared with only carrageenantreated group
Discussion
The present study was performed to investigate the potential anti-inflammatory effects of maprotiline and to elucidate the molecular mechanism(s) involved. The findings of this study evidently showed that maprotiline suppressed the expression of inflammatory mediators both in vitro and in vivo. Maprotiline exhibits strong effects as a norepinephrine reuptake inhibitor with only weak actions on the reuptake of serotonin. Several reports showed that human peripheral blood mononuclear cells as well as the central nervous system possess serotonin and norepinephrine transporter, and might be directly affected by antidepressants [21-23]. Moreover, serotonin and noradrenaline are released from lymphocytes and monocytes [24], and can prompt immunomodulatory properties via receptors that are present on immune cells.
Therefore, we investigated the influence of maprotiline on LPS-induced monocytes to evaluate if the anti-inflammatory effect of maprotiline can be mediated through macrophages. We showed that maprotiline attenuated the expression of iNOS and COX2 in macrophages. iNOS provokes synthesis of NO, which is a free radical molecule and could cause cellular damage in inflammatory sites. On the other hand, COX2 is catalyzing the production of PGE2 from arachidonic acid [25]. In line with our results, Taler and colleagues reported that the expression of COX2 decreased with SSRIs (Selective Serotonin Reuptake Inhibitors) on human T lymphocytes [26]. Moreover, amitriptyline and fluoxetine were shown to attenuate the production of pro-inflammatory cytokine induced PGE2 and NO by cultured human synovial cells [27]. Another study has shown that reboxetine which is a more selective inhibitor of norepinephrine reuptake than maprotiline, decreases NO and IL-6 production [28]. Furthermore, Roumestan and colleagues described that desipramine as an SNRI by affecting gene expression in peripheral cell types including monocytes can decrease the inflammatory mediators [29]. Several other studies also consistently have shown that antidepressants inhibit the NO production [30, 31].
In summary, the data show that maprotiline may reduce the inflammatory responses of monocytes; the mechanism could include a cascade of gene expression secondary to effects on the serotonin transporter that is expressed on the surface of monocytes and lymphocytes.
Inflammation is a complex physiological process, and a variety of cells are involved, so the anti-inflammatory effect of antidepressants cannot be explored just by an in vitro study; therefore we confirmed the anti-inflammatory activity of maprotiline in an in vivo model of carrageenan-induced paw edema. Carrageenan-induced paw edema is a suitable model to evaluate inflammatory mediators involved in acute inflammation. The progress of edema in rat paw following carrageenan injection has been defined as a biphasic event. The primary phase (1 h) of edema has been attributed to the release of histamine, 5-hydroxytryptamine (5-HT) and bradykinin [32]. The second phase (1-6 h) is related to elevated levels of prostaglandins and NO which has been attributed to the induction of inducible COX2 and iNOS. Carrageenan-induced paw edema is neutrophil-dependent. Histological analysis of the subplantar area after carrageenan injection revealed a cellular infiltration mainly by neutrophils. Following the inflammation process, it continues with increase in macrophages infiltration [33].
The findings of this study obviously showed that i.p. injection of maprotiline inhibited development of paw edema over 4 h following carrageenan injection. This result is consistent with our previous reports that showed i.p. injection of maprotiline considerably inhibited paw edema response at 4 h post-carrageenan [14]. We also evaluated the effect of maprotiline on PMN cells migration in inflamed paw tissue and according to our previous study [16], maprotiline inhibited the migration of leukocyte 4 h after carrageenan injection into the site of inflammation.
Moreover, in the present study, the expression of COX2 and iNOS genes were evaluated in the inflamed paw. Studies have shown that activated neutrophils are not the source of iNOS-derived NO and COX2-derived PGs. They are an excellent source of oxygen-derived free radicals which have been implicated in many models of acute inflammation. It has been suggested that resident macrophages in inflamed paw tissue or infiltrating monocytes are the source of COX2 and at 1 h after carrageenan, COX2 mRNA reach a high level of expression [34], whereas iNOS mRNA was detected between 3 and 10 h after carrageenan injection [33]. Similar to our in vitro study on macrophages, the expression of COX2 and iNOS was attenuated by maprotiline in this animal model. The exact mechanism by which antidepressants exert their anti-inflammatory effects remains to be elucidated.
Several studies have demonstrated that some antidepressants increase intracellular concentrations of cAMP through activation of monoamine receptors such as serotonin and noradrenaline receptors [35]. Another in vitro study proposed that the anti-inflammatory effects of various antidepressants on microglia are at least partially mediated by the cAMP dependent protein kinase A (PKA) pathway [28]. In some cell types, it has been shown that cAMP/PKA pathway inhibits the nuclear factor (NF)-κB activity [36], and its activation is identified to induce the expression of iNOS and various pro-inflammatory cytokines in human monocytes [37]. Here, we stimulated macrophages by LPS. Binding of LPS to toll-like receptor 4 (TLR4) activates two principal signaling pathways, which results in activation of the transcription factor, NF-κB, an important upstream modulator for COX2 and iNOS expression [38, 39]. Moreover, the carrageenan used in our in vivo model can induce innate immune pathways of inflammation mediated by TLR4 and BCL10. Carrageenan exposure leads to NF-κB activation by both canonical, involving RelA, p65 and p50, and non-canonical pathway, involving RelB and p52 [40, 41]. Moreover, a number of studies showed that some anti-depressants including SNRIs exert their anti- inflammatory effect by inhibition of NF-κB signaling pathway [28, 29]. Based on these studies and our findings, we suggest that maprotiline evokes its suppressive effect on the expression of COX2 and iNOS by modulating NF-κB pathway. To our knowledge, this is the first study to evaluate the anti-inflammatory effect of maprotiline through the inhibition of COX2 and iNOS gene expression in an in vitro and in vivo model.
In conclusion, the results of this study provide further evidence for the anti-inflammatory effect of maprotiline via inhibition of COX2 and iNOS gene expression based on in vivo and in vitro findings. Further studies are needed to evaluate whether these effects are related to neurotransmitters such as norepinephrine and/or serotonin or not.