Introduction
Severe congenital neutropenia (SCN) is a heterogeneous group of primary errors of immunity which share a common feature of a persistent decrease in neutrophil counts in peripheral blood, usually below 500/µl (0.5 × 109/l) and a maturation arrest of the myelopoiesis in the bone marrow at the promyelocyte/myelocyte stage [1-3]. Patients with SCN are constantly at risk of life-threatening bacterial infections, and occasionally of fungal infections [2-5]. As the disease progresses, the risk of developing the myelodysplastic syndrome and acute myeloid leukemia increases [1-3, 5]. The prevalence of SCN is estimated at 1-2 : 1,000,000 live births [1, 5].
In 1950, the Swedish physician Rolf Kostmann described a family suffering from an autosomal recessively inherited disease with severe neutropenia and recurrent bacterial infections since early childhood [6]. Since then, genetic studies have identified several gene defects in patients with SCN including the neutrophil elastase (ELANE) gene, HCLS1 associated protein X-1 (HAX1) gene, glucose-6-phosphatase catalytic subunit 3 (G6PC3), growth factor independent protein 1 (GFI1), and vacuolar protein sorting-associated protein 45 (VPS45) [2]. These genetic defects are characterized by impaired maturation of neutrophil granules [2]. In 2014 Boztug et al. published the first description of jagunal homolog 1 (JAGN1) mutations in patients affected with SCN [7]. JAGN1 is required for granulocyte colony-stimulating factor receptor-mediated signaling and is necessary in the differentiation and survival of neutrophils [7, 8].
In the 1950s, the mortality rate from infections in severe congenital neutropenia was 90% [2]. The 1980s brought a breakthrough in the treatment of chronic neutropenia due to the introduction of recombinant granulocyte colony-stimulating factor (rHuG-CSF) for clinical use [2, 9, 10]. For patients with SCN not responding to treatment with subcutaneous G-CSF, the only currently available therapeutic option is hematopoietic stem cell transplantation (HSCT) [3, 5, 11, 12].
The clinical signs of congenital neutropenia include delayed separation of the umbilical cord stump, inflammation of the navel, recurrent upper and lower respiratory tract infections, oral ulcers or mucositis, chronic gingivitis, skin infections such as abscesses or bacterial cellulitis, bacterial lymphadenitis, bacterial septicemia, and urinary tract infections [1-3, 13-16].
The most common microorganisms causing infections in patients with SCN are Gram-positive bacteria – Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes – and the second most frequent are Gram-negative bacilli Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae [1, 4, 5, 13]. Anaerobes constituting the flora of the oral cavity – Peptococcus, Peptostreptococcus, Bacteroides fragilis, and Fusobacterium spp. – can also become a causative factor of severe infection in patients with SCN [15].
Case description
A boy of Roma origin, the son of consanguineous parents, was admitted at the Children’s Memorial Health Institute in Warsaw at 2 years of age due to chronic neutropenia.
His absolute neutrophil count (ANC) varied from 0.06 to 0.6 × 109/l and he presented with high monocytosis in peripheral blood. He suffered from recurrent, severe bacterial infections, chronic gingivitis and painful mouth ulcers. Bone marrow examination showed, typical for SCN, maturation arrest at the myelocyte stage. Because of low height (below 10 percentiles for age) and failure to thrive, Shwachman-Diamond syndrome was considered, but molecular diagnostics did not reveal any SBDS gene mutations. G-CSF treatment was initiated, and some improvement was observed, but his ANC increased to 1.0 × 109/l only after high doses of G-CSF (approx. 40 µg/kg/d). After a few months, G-CSF-treatment had to be stopped due to poor tolerance (bone and muscle pain). Instead, the child was put on antibiotic prophylaxis and vaccinated against encapsulated bacteria. Eventually, HSCT was considered, but his parents did not approve such a treatment. With time, his height and weight increased over 25 percentiles. At first, molecular studies excluded defects in the ELANE and HAX1 genes, but finally homozygous mutations in JAGN1 were identified. Currently the boy is doing well on antibiotic prophylaxis. Despite profound neutropenia he had no severe infections for many years.
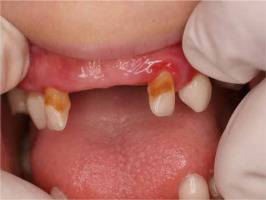
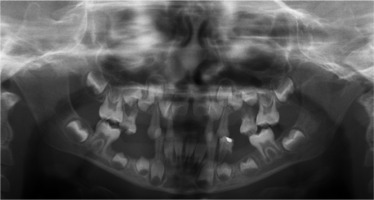
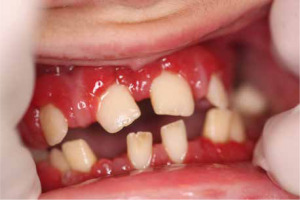
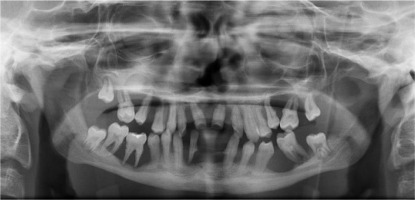
When he was 5 years of age the intraoral examination revealed incomplete primary dentition with five missing teeth, marginal gingivitis, interdental papilla hypertrophy, carious lesions in seven primary teeth, and one permanent tooth of various degrees of advancement for conservative treatment (Fig. 1). The patient was qualified for simultaneous dental treatment under general anesthesia. A panoramic radiograph picture was taken showing the advanced destruction of the alveolar bone in the maxilla and the alveolar part of the mandible. Three primary teeth were completely devoid of bone base. The roots of the lower incisors were only half of their normal length (Fig. 2). Under antibiotic protection (amoxicillin with clavulanic acid at a dose of 30 mg/kg), professional removal of dental plaque and conservative treatment of teeth extraction of four primary teeth with complicated caries and periodontitis were performed. The patient was provided with permanent dental care.
During follow-up visits symptoms of gingivitis and periodontitis were observed.
Every time oral hygiene instruction was provided and professional oral cavity hygiene and conservative treatment of cavities were performed under antibiotic prophylaxis. The patient was recommended to use topical chlorhexidine gel 2-3 times a day for 10 days and to rinse the mouth with a preparation containing octenidine twice a day for 10-14 days.
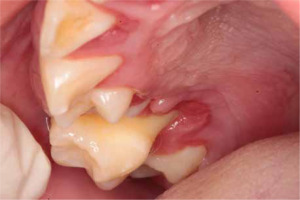
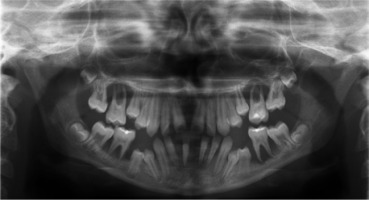
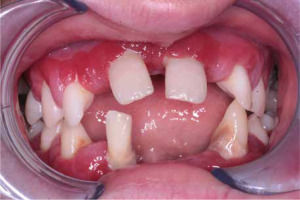
When the patient was 10, the exposure of two-thirds of palatal roots and third-degree loosening of teeth were observed (Fig. 3). A panoramic radiograph picture was taken (Fig. 4), which revealed complete absence of the bone base in all permanent first molars. Teeth 16 and 26 were qualified for extraction, which was performed in an outpatient setting under local anesthesia and antibiotic treatment (clindamycin, 10 mg/kg/dose). The obtained material from granulomatous lesions was submitted for histopathological examination, the results of which indicated non-specific inflammatory granulation tissue.
The next year, tartar deposits in the incisors of the mandible and gingivitis were discovered, and the mandibular first molars and central incisors showed signs of second-degree loosening. The patient was recommended to continue conservative treatment and undergo removal of the tartar deposits under antibiotic treatment (amoxicillin with clavulanic acid, 2 doses of 30 mg/kg with an interval of 12 hours, given at home). A few months later (Fig. 5), examination revealed intensified inflammation around tooth 36 with its roots halfway exposed, as well as looseness of teeth 32-42. Extraction of tooth 36 was performed under local anesthesia in an outpatient setting and covered with intravenous antibiotic (amoxicillin with clavulanic acid 30 mg/kg/dose).
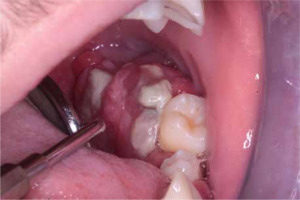
At the age of 15 (Fig. 6), the patient came to the Children’s Dentistry Clinic of the Children’s Memorial Health Institute due to an exophytic lesion near teeth 37 and 38 on the lingual side (Fig. 7). During the intraoral examination, a lesion measuring 25 mm was found; it had a wide base and was elastic and hard, covered with a smooth mucosa with a vivid red color, and was not painful. Submandibular lymph nodes on the left side were enlarged, albeit not painful; they were movable against the skin and the base. All teeth were also found to be loose, with deep periodontal pockets and inflammation of the marginal periodontium. A control panoramic radiograph picture was taken (Fig. 8). The patient was qualified for surgery to remove the lesion and extract twenty-one teeth. Despite agranulocytosis, after an immunologist’s consultation, the patient underwent the procedure under general anesthesia and antibiotic treatment (clindamycin intravenously, 10 mg/kg/dose). An exophytic lesion was removed from the alveolar part of the mandible. The sockets and the tumor bed were secured with a collagen sponge and approach sutures. The histopathologic examination of the collected material revealed giant cell epithelium; as for the material taken from the alveoli, its fragments were covered with partially dilated multilayer squamous epithelium without any signs of atypia.
The numerous extractions and damage to the alveolar process after the removal of the lesion had a negative impact on the emotional state of the patient and required treatment to restore the aesthetics of the dentition. The pa- tient was referred to the Department of Prosthodontics of the Medical University of Warsaw to have his lost chewing function restored and pronunciation and dental aesthetics improved. Due to the condition of the prosthetic base, the patient was offered removable dental prostheses. At the time of the prosthetic procedure at the Department of Prosthodontics, Medical University of Warsaw, the patient came to the Children’s Dentistry Clinic of the Institute of Pediatric Medicine due to a painful traumatic lesion – a pressure ulcer – on the lower lip mucosa around teeth 33-34. Topical application of orthodontic wax on teeth 33 and 34 was recommended. Biostimulation laser therapy with a wavelength of 635 nm and at a dose of 4 J/cm/20 s was performed as supportive treatment. The biostimulation laser therapy significantly reduced pain and shortened the lesion healing time. The patient remains under the constant care of the Dental Surgery Clinic of the Institute of Pediatric Medicine and the Prosthetics Department of the Medical University of Warsaw.
Discussion
Neutropenic patients with ANC decreased below 500 cells/µl are vulnerable to bacterial infections, which may be life-threatening but usually respond well to modern antibiotics. Another common problem of SCN patients who have not undergone HSCT is periodontitis, which may lead to both infections and permanent teeth loss. A recent study of Rotulo et al. showed that approx. 30% of adult patients with ELANE deficiency in the French Severe Chronic Neutropenia Registry present with edentulism [17]. The pathogenesis of gingivitis and periodontitis is multifactorial; it includes interactions between oral microbiota and host defense [18]. Neutrophils are the key cells for oral cavity health and neutrophil deficiency or dysfunction usually results in periodontal disorder [18, 19]. The literature review points to the conclusion that pathological symptoms in the oral cavity in patients with SCN usually start at 2 years of age and become a serious, life-long problem despite rHuG-CSF treatment [13-17, 19]. The literature data indicate that in 20% of patients with congenital neutropenia, ulceration lesions located in various parts of the oral cavity may be symptoms of the disease [16]. Advanced periodontitis, severe gingivitis, and significant alveolar bone loss resulting in the premature loss of teeth – both in the case of primary and permanent dentition – may indicate a severe disturbance in the production and release of neutrophils from the marrow into the peripheral blood [5, 15-17]. In our patient, oral cavity changes typical for SCN – in the form of gingivitis, i.e. swelling, redness, and bleeding from periodontal tissues – appeared when he was 2 years and led to the premature loss of several primary teeth.
Dental management in such patients consists in regular dental visits combined with oral hygiene instruction, health education, professional dental cleaning procedures, and the use of antibacterial gels and rinses. In cases of advanced periodontal disease, it is necessary to extract teeth under antibiotic treatment. The patient described in this case report suffered from significant hygiene negligence caused by pain and bleeding of the gums during hygienic procedures which, coupled with the lack of regular follow-up visits and severe congenital neutropenia, led to the premature loss of dentition. Despite being 15 years old, he had only 5 teeth left, all of them affected by significant progression of periodontal disease. As such, it was necessary to provide him with a removable dental prosthesis.
According to Segel and Halterman and Olczak-Kowalczyk et al., the most effective way to prevent oral inflammation and premature tooth loss is to motivate the patient to maintain proper oral hygiene [1, 15]. Regular and frequent dental checkups and professional prevention of caries and periodontal disease reduce the severity of chronic periodontitis and the risk of caries [15].
Interestingly, besides neutropenia, patients affected with SCN also present with deficiency of neutrophil granule-associated proteins such as antimicrobial peptides cathelicidin LL-37 and human neutrophil peptides 1-3 [19, 20]. The concentration of these anti-inflammatory peptides in patients’ saliva does not increase even during successful treatment with G-CSF preparations [19]. Therefore, chronic periodontitis occurs in patients with SCN who have not undergone HSCT. Though an attempt was made to treat our patient with G-CSF, the high doses that he required to raise his ANC above 1000/µl contributed to the occurrence of side effects and resulted in treatment cessation. After the diagnosis of SCN type 6 (caused by the JAGN1 gene mutations) was confirmed by molecular examination, the boy was offered HSCT treatment, which was not implemented due to the parents’ objection.
Conclusions
The presented literature and the described case report confirm that oral mucosa lesions in patients with SCN are often infectious and may lead to systemic infection and life-threatening conditions. They manifest as erosions, ulce- rations, and hyperplastic changes in the mucosa. Their most characteristic symptoms, however, are gingivitis and rapid periodontal destruction not amenable to local treatment. Attention to oral hygiene, the use of local antibacterial agents, and regular visits to the dentist’s office can alleviate the course of the disease process in the periodontal tissues and prevent the formation of odontogenic infection foci. In the case of procedures related to the disruption of tissue continuity and bacteremia, such as scaling or tooth extraction, the following antibiotic prophylaxis is recommended: amoxicillin with clavulanic acid administered in a single dose of 50 mg/kg, and in children allergic to penicillin, clindamycin in a single dose of 20 mg/kg.