INTRODUCTION
Myopia is a refractive vision disorder presenting with blurry distance vision and good near vision. Myopia is usually classified into three types: refractive, axial, and mixed. In refractive myopia, the overactive ocular optical system causes light rays entering the eye to refract not on the retina but in front of it, resulting in a blurred image forming on the macula. In axial myopia, the eye is too long, causing light rays to be refracted in front of the retina. Mixed myopia refers to a combination of both refractive states described above.
The etiopathogenesis of myopia involves both genetic and environmental factors. Currently, an increasing focus is directed towards the influence of environmental factors such as prolonged near work, excessive use of smartphones and laptops without breaks, in inadequate lighting conditions, and decreasing time spent outdoors during daylight hours.
The treatment options for myopia are varied. However, there is no therapeutic modality that is 100% effective in halting the progression of a developing refractive error. When discussing the treatment options for myopia, it is important to highlight that the final decision rests with the ophthalmologist who adapts the therapeutic approach to the patient, considering factors such as their age, the onset of the refractive error, and the annual rate of progression.
Since the turn of the 21st century, there has been a concerning rising trend in the prevalence of myopia worldwide. The latest statistics are particularly alarming in Central and Eastern Asia, where over 90% of young adults are affected by this visual impairment, compared to approximately 50% in the USA and Europe. In 2000, it was estimated that 22.9% of the global population had myopia, with 2.7% having high myopia. However, projections suggest that by 2050, these figures will rise to 49.7% and 9.8%, respectively [1]. This implies that approximately one billion people will be affected by high myopia. Hence, delaying the onset of myopia and slowing its progression in school-age children may be crucial in reducing the prevalence of high myopia later in life [2].
ROLE OF PHYSICAL ACTIVITY IN THE PREVENTION OF MYOPIA IN CHILDREN
Time spent outdoors has a significant inhibitory effect on the progression of myopia through various mechanisms. These include higher ambient brightness, reduced peripheral blur, elevated vitamin D levels, different chromatic spectrum of outdoor light, increase in physical activity, reduced near work, stability of the circadian rhythm, and different spatial-frequency characteristics [3-5].
Observational studies conducted in Copenhagen found that adolescents who declared engaging in physical activity for less than three hours per day or spending more than six hours daily using computers were at double risk of myopia compared to adolescents devoting more time to physical activity and spending less time in front of screens [6, 7]. Furthermore, research from the UK revealed a significant correlation between both the level of physical activity and the duration spent outdoors and the occurrence of myopia. Importantly, spending time outdoors was found to have a greater impact than engaging in physical activity [7]. Sanchez-Tocino et al. achieved comparable findings. Their study, isolating the impact of physical activity and outdoor time on changes in spherical equivalent refractive error, showed that engaging in sports did not significantly reduce the progression of refractive error, as opposed to spending ample time outdoors [8]. The authors determined that increasing outdoor time to 11 hours or more per week decreased the risk of myopia by one-third.
A reduced risk of myopia in physically active children and adolescents, compared to a group that does not engage in regular exercise, was also reported in Russian studies [9]. Physical activity was found to stabilize visual acuity and slow down the progression of low-to-medium myopia [9]. A recent multicenter study showed that increasing the amount of time spent outdoors by one to three hours a day could reduce the risk of myopia by 50% [10]. Moreover, a 2012 metaanalysis indicated a 2% decrease in the risk of developing myopia with each additional hour spent outdoors per week [11]. Nevertheless, further research is needed to determine the time and type of activity that may inhibit the development and progression of myopia.
OPTICAL METHODS FOR MYOPIA CORRECTION
Conventional approaches, which involve wearing spectacles with concave monofocal lenses or soft contact lenses, correct the refractive error effectively but do not contribute to the elongation of the eye. Bifocal or progressive spectacles are also used to reduce the accommodative effort for near vision.
ORTHOKERATOLOGY
Orthokeratology is an alternative treatment for myopia. The method involves the use of specially designed rigid gas-permeable contact lenses which apply pressure to the cornea, altering its curvature, which leads to corneal flattening and, consequently, a reduction or elimination of myopia [12].
Unlike traditional orthokeratology lenses, which had a series of progressive rings that flattened the corneal periphery while matching the central part to the corneal curvature, new-generation orthokeratology lenses, employed in recent years, use what is known as inverted geometry. In this design, the central part of the lens is flatter compared to the natural corneal curvature, with surrounding steeper peripheral zones [13]. This facilitates appropriate lens centration. Orthokeratology contact lenses are worn solely at night and taken out in the morning. The alteration of corneal curvature (flattening) induced by the mechanical pressure from the lens occurs due to the redistribution of the epithelium and anterior part of the corneal stroma across its central area spanning about 5-6 mm in diameter [14]. This allows for clear daytime vision without the need to use spectacles.
Studies show a decrease in axial length growth of the eye in long-term orthokeratology lens wearers compared to the control group. The slowdown in the elongation of the eye is most likely to be due to mechanical changes. Orthokeratology flattens the central cornea, ensuring that a clear image is formed in the central part of the retina. The peripheral cornea is steeper, resulting in myopia in the outer peripheral regions of the retina. It has been suggested that the degree of paracentral and peripheral myopia impacts the elongation of the eye [15]. Under normal conditions, the retinal circumference in myopic patients is hyperopic because of the increased anterior-posterior elongation of the eye compared to the equatorial length. A similar slowdown in ocular elongation is observed in patients wearing soft multifocal contact lenses with peripheral positive power. It is hypothesized that in both types of lenses, the mechanism of slowing down the elongation of the eye, and consequently increasing myopia progression, is similar and involves the of peripheral myopia.
A challenge associated with orthokeratology is the unpredictability of refractive error improvement (reduction) due to improper centration of the lens. Another issue is the recurrence of the defect and the return of eye elongation once orthokeratology treatment is discontinued.
The increasing applications of orthokeratology for treating myopia in children is associated with a risk of complications. Prolonged use and nighttime wear may result in chronic corneal hypoxia even with the use of gas-permeable materials. The most serious complications of orthokeratology include bacterial keratitis and corneal ulceration. Fortunately, these complications are rare, though they occur more frequently in individuals treated by orthokeratology compared to patients wearing daytime multifocal soft lenses for myopia treatment [16]. Also, patients treated with orthokeratology lenses more commonly experience corneal staining (damage to the corneal epithelium). Furthermore, a decrease in contrast sensitivity may be observed. Chronic orthokeratology lens users also experience reduced tear secretion and decreased tear film stability. Long-term wearers of orthokeratology lenses are more prone to developing pigmentary changes in the corneal epithelium, specifically iron deposits, which, however, are clinically insignificant [12]. Patients undergoing orthokeratology treatment exhibit a slight thinning of the central part and a slight thickening of the peripheral parts of the cornea, along with shallowing of the anterior chamber. Considering the aforementioned factors, including nighttime use and chronic wearing of orthokeratology lenses, there are concerns regarding the short- and long-term effects of such treatment on the condition of the corneal endothelium. Currently, there is no evidence indicating any adverse short-term impact of these lenses on the corneal endothelium. Nevertheless, the possibility of changes occurring in the corneal endothelium in chronic wearers of orthokeratology lenses over time cannot be ruled out.
MECHANISM OF SPECTACLE LENSES WITH DEFOCUS INCORPORATED MULTIPLE SEGMENTS TECHNOLOGY
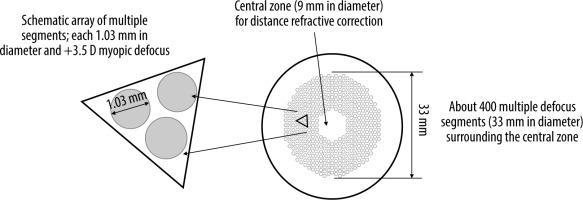
Given the unsatisfactory outcomes or challenges encountered with certain methods aimed at inhibiting myopia progression, there is a continuous pursuit of new solutions [17]. One of them is based on innovative spectacle lenses with Defocus Incorporated Multiple Segments (DIMS) techno- logy. The patented multi-segment defocus technology was developed by Hong Kong researchers in 2014. The DIMS lens consists of a central optical zone (9 mm in diameter) for correcting the refractive error and ensuring clear vision, and a surrounding annular therapeutic zone (33 mm in diameter) comprising multiple small segments with microlenses with a power of +3.50 diopters (D). The diameter of each segment is 1.03 mm (Figure 1) [18].
Figure 1
The design of the defocus incorporated multiple segments (DIMS) spectacle lens [18]
Source: Lam CSY, Tang WC, Tse DY i wsp. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol 2020; 104: 363-368.
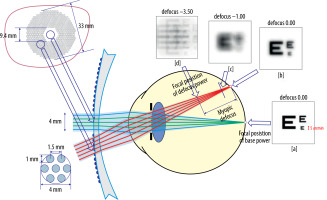
DIMS spectacle lenses guarantee clear vision at all distances, while also inducing peripheral myopic defocus on the retina. Viewing an object through the peripheral part of the lens results in the appearance of multiple focal points in front of the retina. Depending on the relative refractive error, these are perceived as blurry images. The alternating arrangement of regions ensuring normal visual perception with zones of myopic defocus allows maintaining full visual acuity, while simultaneously controlling the progression of myopia (Figure 2) [18, 19].
Figure 2
Retinal images after light rays pass through a lens with DIMS technology [19]
Source: Lam CSY, Tang WC, Qi H i wsp. Effect of defocus incorporated multiple segments spectacle lens wear on visual function inmyopic Chinese children. Transl Vis Sci Technol 2020; 9: 11.
The mechanism underlying the design described above is peripheral myopic defocus. As reported in the literature, numerous studies conducted to date in animals demonstrate the crucial role of this mechanism in inhibiting ocular elongation. The findings of the mentioned studies can be summarized as follows:
optical defocus on the retina plays a role in regulating eye growth and the progression of refractive error;
the presence of local vision-dependent mechanisms suggests that peripheral vision may impact eye shape and axial length in a manner independent of central vision;
visual signals from the fovea are not essential for many aspects of vision-dependent eye growth;
when conflicting signals arise between the central and peripheral retina, peripheral visual signals may take precedence in determining the central progression of the refractive error;
refractive errors may change with eccentricity, and peri- pheral defocus may alter central refractive development [20].
A number of studies have investigated the links between near work and myopia progression, revealing that intensive near-visual work leads to high accommodative effort. This can result in an inadequately strong accommodative response when focusing on nearby objects. A consequence of this process is the formation of hyperopic defocus on the retina during near visual work. This observation has prompted the theory that the optical blurring of the image on the retina caused by an impaired accommodative response might be a signal triggering excessive eye elongation, leading to progressive myopia in children and adolescents [21].
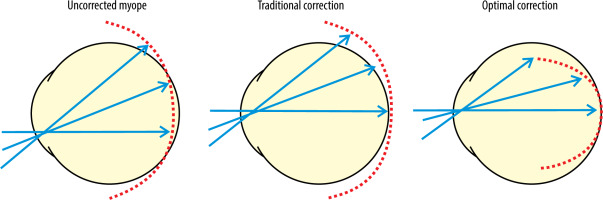
In standard single vision spectacle lenses used to correct myopia, peripheral light rays focus precisely behind the retina, creating hyperopic defocus, which prompts elongation of the eye. The purpose of DIMS lenses is to eliminate peripheral visual signals that may stimulate eye growth and to generate visual signals that will restrict ocular elongation, thereby inhibiting the process of myopia progression (Figure 3) [20].
Figure 3
Focusing of light rays on the retina of the myopic eye depending on the correction method used [20]
To verify the efficacy of the proposed solution, a study was conducted, with the results published in the “British Journal of Ophthalmology” in 2020 [19]. The outcomes of the study indicate that the innovative DIMS lenses used by myopic children to slow down the progression of myopia as compared to the control group of children wearing conventional spectacle lenses. The clinical trial was carried out in 183 children aged 8-13 years, with the mean spherical equivalent refraction of –2.93 D in the experimental group and –2.70 D in the control group. The mean axial length of the eye was 24.85 mm and 24.72 mm, respectively. Myopia in the children included in the study ranged between –1.00 and –5.00 D, and astigmatism was ≤ –1.50 D. The children were randomly assigned to the study group or the control group. After a two-year follow-up, 85% of patients from the study group and 90% from the control group completed the experiment. The change in refraction was –0.41 ±0.06 D in the DIMS lens group and –0.85 ±0.08 D in the control group. The difference in axial elongation of the eye was 0.21 ±0.02 mm and 0.55 ±0.02 mm in the DIMS and control groups, respectively. Analyzing the entire study population, it was found that in children wearing DIMS spectacle lenses myopia progressed nearly 50% more slowly and the change in axial length was reduced by 62% compared to patients using conventional single vision spectacle lenses. It was also noted that in 21.5% of patients there was no increase in refractive error [19, 22].
Vision correction using multiple-segment (DIMS) spectacle lenses was found to have significantly slowed myopia progression in school-aged children compared with single vision spectacle lenses. The outcomes of the study are highly promising, particularly given the fact that wearing spectacles is a safe method widely accepted by parents. The results described above were obtained after a 2-year follow-up study in Chinese children. Therefore, further research involving participants from outside this region is necessary to assess the efficacy of the solution in the broader population of children.
ATROPINE IN THE PREVENTION OF MYOPIA PROGRESSION
Atropine is a tropane alkaloid that acts as an antagonist of postganglionic muscarinic receptors M1 and M2. It has a wide range of applications in various fields including cardiology and anesthesiology. In ophthalmology, atropine is prescribed for the treatment of iritis and cyclitis to achieve prolonged pupillary dilation and prevent the formation of posterior synechiae. Atropine is also used to temporarily paralyze the ciliary muscle before evaluating ocular refraction in young children.
This non-selective muscarinic antagonist inhibits the progression of myopia. The mechanism by which atropine inhibits myopia is not completely understood. It does not work solely by inhibiting accommodation because it is known to halt myopia in chickens, which lack accommodation ability. This suggests a non-accommodative mechanism of atropine action, possibly through the activation of nicotinic receptors. By acting on M1/M4 receptors in the retina (likely within amacrine cells), atropine can potentially inhibit ocular elongation through a cascade of chemical neurotransmitters. According to an alternative theory, atropine exerts a direct inhibitory effect on the synthesis of glycosaminoglycans by scleral fibroblasts, without muscarinic receptor involvement [23]. Based on yet another theory, an increased amount of light falling on the retina as a result of pupillary dilation following the administration of atropine has a direct inhibitory effect on eye elongation [24].
The first studies exploring the potential benefits of atropine for myopia control (ATOM 1) showed that applying 1% atropine solution in the form of eye drops slowed down eye elongation and, consequently, myopia progression, yet it was associated with side effects related to accommodation paralysis and pupil dilation [25]. Another study (ATOM 2) compared the effects of atropine drops at different concentrations (1%, 0.5%, and 0.01%) [26]. It was found that 0.01% atropine caused the least side effects while demonstrating comparable efficacy to 1% atropine in controlling myopia progression. The study authors concluded that using eye drops containing 0.01% atropine was more effective in slowing down the progression of myopia in children compared to higher concentrations of atropine, without elevating the risk of ocular adverse effects like photophobia or near vision issues. The recent LAMP study (2020) confirmed that atropine at low concentrations reduced spherical equivalent progression and eye elongation simultaneously [24]. The results showed that low-concentration atropine drops administered once daily before bedtime for up to two years was an effective, safe, and well-tolerated method of preventing myopia progression in children. According to a WHO report on myopia, 0.01% atropine is currently the most common approach for myopia control in children in Asian countries such as Singapore, where eye drops with 0.01% atropine (Myopine) are commercially available [27]. Research from North America and Europe also provides evidence for the efficacy of 0.01% atropine in halting myopia progression [28, 29].
LASER CORRECTION OF MYOPIA AND MYOPIC ASTIGMATISM IN CHILDREN
Stabilization of refractive error occurs after puberty and continues until around the age of 20; this applies to hyperopia, myopia, and astigmatism alike. Consequently, surgical treatment of myopia in children is contraindicated. However, there are certain exceptions where surgical intervention may be recommended, especially in children with unilateral visual impairment and high degrees of anisometropia. Differences in refractive error above 3.0 Dsph and above 1.5 Dcyl pose challenges in selecting spectacle correction. If contact lens intolerance is present concurrently, it can lead to the development of amblyopia. This, in turn, may be the underlying cause for the lack of development of binocular vision, as anisometropia and associated aniseikonia present barriers to the development and maintenance of fusion [30]. Spectacle correction frequently proves inadequate in correcting significant anisometropia. The risk of amblyopia in children with anisometropia is higher when the defect is present in one eye compared to when it affects both eyes. Since the introduction of laser refractive surgery, which was found to be effective and safe in adults, it has been increasingly employed in young patients. Laser refractive surgery is a method of correcting refractive errors by modifying the anterior curvature of the cornea through the use of an excimer and/or femtosecond laser (flattening of the central cornea for myopia correction). Refractive techniques are registered and approved by regulatory authorities for medicinal products and medical technologies in both Europe and the United States [31-34]. The following modalities are approved for the correction of myopia:
LASIK/Femto-LASIK (laser-assisted in situ keratomileusis) – up to –10.0 D (–14.0 according to Food and Drug Administration [FDA]) without astigmatism or up to –5.0 D (SE –10.0 D) with astigmatism,
LASIK wavefront-guided – up to –8.0 D without astigmatism or up to –4.0 D (SE –8.0 D) with astigmatism; with aberrometric measurements,
LASIK topography-guided – up to –8.0 D without astigmatism or up to –3.0 D (SE –9.0 D) with astigmatism; with topographic measurements of the cornea,
PRK/LASEK (photorefractive keratectomy/laser subepithelial keratomileusis) – up to –10.0 D (–12.0 according to FDA) without astigmatism or up to –4.0 D with astigmatism (SE –10.0 D),
SMILE (small incision lenticule extraction) – up to –10.0 D without astigmatism or up to –5.0 D (SE –10.0 D) with astigmatism.
WhileFDA approval does not extend to laser refractive procedures in patients under 18 years of age, photorefractive keratectomy (PRK) is considered acceptable in specialized centers for the treatment of children with high anisometropia or severe visual defects not correctable by conventional methods who are at risk of developing amblyopia.
Complications following laser correction of myopia are not more prevalent in children compared to adult patients. Prolonged epithelialization, decentration of the photoablation zone, haze, epithelial ingrowth under the flap, and the presence of Bowman’s membrane folds have been reported [33, 34]. Because of potential problems with patient cooperation, short-term general anesthesia or light sedation is often administered. It has been observed that in children aged 12 and older the course of the procedure is similar to that in adult patients. However, it is important to recognize that children after refractive surgery require slightly modified management. This is due to the topical application of steroid preparations; thus, long-term monitoring of intraocular pressure (IOP) and prophylactic use of IOP-lowering drops are required. It is recommended to monitor refractive error in children through the measurement of axial length of the eye and corneal topography to be able to distinguish between the progression of the defect and the regression of the treatment effect. Before proceeding with the procedure, it is important to thoroughly inform parents, legal guardians, and the patient about the indications and contraindications involved.
SURGICAL TREATMENT FOR PROGRESSIVE MYOPIA – POSTERIOR SCLERAL REINFORCEMENT
High myopia is usually defined as a spherical equivalent of > 6.0 D. High myopia is associated with pathologic myopia. As myopia progresses, excessive axial elongation of the eye occurs, resulting in pathological scleral thinning and distortion. An outpouching of a circumscribed region of the posterior pole, referred to as posterior staphyloma, develops, accompanied by atrophic and degenerative changes to the choroid and retina. Posterior scleral staphyloma is one of the pathological hallmarks of high myopia, and one of the primary contributors to the onset of macular complications, including myopic maculopathy, myopic choroidal neovascularization, and even macular retinoschisis. It may also induce optic neuropathy and potentially result in blindness.
Surgical treatment options for progressive myopia encompass laser refractive surgery of the cornea, implantation of a posterior chamber phakic intraocular lens, and posterior scleral reinforcement. In terms of its effect on the axial length of the eye, posterior scleral reinforcement is considered the sole most effective approach by surgeons using this method. In recent years, scientists have also proposed the technique of subscleral injection of mesenchymal stem cells and dopamine injection as potential treatments for high myopia. These methods represent a promising new strategy to halt the progression of myopia [35].
Procedures reinforcing the posterior pole of the eye are designed to inhibit the elongation of the ocular axis. Posterior scleral reinforcement surgery using either biological or nonbiological materials involves strengthening the weakened scleral region near the posterior pole, thereby impeding the continuous increase of the axial length of the eye. The approach was initially proposed by Shevelev in 1930, with subsequent modifications by Snyder and Thompson in 1972 [36].
Mechanism of posterior scleral reinforcement
Based on experimental animal studies, histopathological changes following posterior scleral reinforcement surgery are categorized into four phases: inflammatory reaction period (1-2 weeks after surgery); granuloma formation stage, angiogenesis stage (2-4 weeks after surgery); collagen fiber-formation stage (1-3 months after surgery); and connective tissue proliferative stage (> 3 months after surgery) [35]. In the early period after surgery, the inflammatory response and the dissolution of collagen fibers are observed. Almost at the same time, the process of repair of the implanted sclera is initiated. Neovascularization appearing on the surfaces of the donor and recipient sclera and between them about one week after surgery grows deeper into the recipient sclera over time, reaching a peak in the posterior pole of the eye one to three months postoperatively. Then, the inflammatory response resolves completely [35]. Neovascularization improves the nutritional status of the posterior pole in high myopia, thus improving the patient’s visual function [37]. PSR has been shown to improve blood flow in the central retinal artery and choroid in patients with high myopia. Following a long period of repair and reconstruction, the implanted scleral graft finally fuses with the recipient sclera. The scleral thickness increases significantly, and so does the hardness, thereby achieving the objective of mechanically reinforcing the sclera.
Types of posterior scleral reinforcement
Numerous clinical studies have demonstrated that among various surgical methods of posterior scleral reinforcement, single-band posterior scleral reinforcement is the safest and most effective procedure for treating progressive myopia [35-37]. In the assessment of eligibility for surgery, an increase in myopia of more than 1 D per year or an increase in the length of the ocular axis of more than 1 mm is taken into consideration [38]. Prior to the procedure, an accurate assessment of the fundus perimeter is required.
The most commonly observed complications after PSR include bulbar conjunctival edema, corneal dellen, ocular hypertension, transient diplopia, ocular abduction weakness, rejection of the reinforcing material and, rarely, retinal detachment [36-39].
In conclusion, surgical procedures modifying the remodeling of the posterior scleral pole (PSR), causing direct mechanical reinforcement of the bulbar wall, are particularly important for slowing down the progression of high myopia. Scleral reinforcement treatment is deemed safe and effective in stabilizing vision, preventing elongation of the ocular axis, and halting further progression of myopia. However, some researchers believe that it does not yield satisfactory results in all cases. As a result, only a few centers perform the procedure.
CONCLUSIONS
New-generation spectacle lenses with myopic defocus are believed to slow down the progression of myopia by 60%. The efficacy of atropine treatment is similar, with approximately 59% myopia inhibition, compared to 50% for contact lenses. The least favorable outcomes are associated with orthokeratology, with only 45% of patients achieving inhibition of the defect.
When discussing available treatment options for myopia, it is important to note that the final decision rests with the ophthalmologist who adapts the therapeutic approach to the patient, considering factors such as their age, the onset of the refractive error, and the annual rate of progression.
It is important to recognize that reducing the global burden of myopia necessitates educating not only ophthalmologists but also general practitioners, healthcare professionals, patients and their families, as well as local and national public health institutions.

 POLSKI
POLSKI




