Introduction
Nocturnal enuresis (NE) is urinary incontinence during sleeping, occurring in a child who has already completed toilet training or in children ≥ 5 years old [1, 2].
There are two types of enuresis: monosymptomatic without any other lower urinary tract symptoms and without a history of bladder dysfunction (approx. 80% of children with NE); and nonmonosymptomatic (NMNE), associated with symptoms from the lower urinary tract such as daytime incontinence, urgency, frequent or infrequent micturition, syndrome of squatting, urinary tract infection, etc. (approx. 20% of children with NE) [1].
Primary nocturnal enuresis occurs in children who have never achieved a satisfactory period of nighttime dryness, whereas secondary nocturnal enuresis occurs in children who develop enuresis after a dry period of at least six months (most often in children with congenital anomalies of the urinary tract, or psychiatric disorders) [1].
In the pathogenesis of primary monosymptomatic nocturnal enuresis (PMNE), nocturnal polyuria, associated with inadequate secretion of antidiuretic hormone at night, nocturnal detrusor overactivity and sleep disorders play the most important roles [1-3]. In the treatment of PMNE in children, depending on the cause, desmopressin, bed-wetting alarms, anticholinergics or sometimes tricyclic antidepressants are used [1-3].
Copeptin (CT-proAVP), also called the arginine-vasopressin-companion protein, is a 39-amino acid polypeptide, which is a C-terminal fragment of pre-provasopressin [4]. It is believed that the copeptin may play a role in the formation of the proper structure of pre-provasopressin, necessary for arginine vasopressin (AVP) hormone maturation [5]. In response to osmotic, volumetric and stimuli from baroreceptors, enzymatic cleavage of prohormone to the active form of vasopressin occurs [5, 6]. AVP is secreted to the circulation in a pulsative mode and is quickly eliminated with serum with in vivo half-life of 24 minutes [6]. Assessment of circulating AVP was found to be difficult and burdened with a large laboratory error, because the AVP molecule is unstable both in vivo and in vitro, and decomposes rapidly in blood samples [6].
Over 90% of blood AVP is bound to thrombocytes, which causes falsely increased AVP concentration in plasma and serum. On the other hand, incomplete separation of plasma samples and their contamination with platelets or prolonged storage of collected blood samples may result in a decreased concentration of AVP [6].
In contrast to AVP, copeptin has a long half-life and does not bind to platelets as AVP. It is present in plasma in much higher concentrations compared to AVP [6], but still its level correlates closely with plasma AVP levels [4]. Currently, copeptin is considered as a clinically useful substitute marker for AVP [7].
In recent years, numerous studies evaluating serum copeptin level in different clinical conditions were published e.g. in hyponatremia [8], polyuria [9], metabolic syndrome [10], obesity [11], pneumonia [12], chronic kidney disease [13, 14], hypertension [15] and septic shock [16]. Single reports assess copeptin in children with nocturnal enuresis [17, 18]. Nalbantogˇlu et al. found that patients with nocturnal enuresis had significantly lower copeptin compared to healthy peers [17] and Hara et al. revealed that plasma copeptin day/night ratio before treatment was predictive of desmopressin response [18]. The position of copeptin as a reliable marker of PMNE is still under debate and there are no data on the relation between copeptin level and clinical (e.g. bladder capacity) and biochemical parameters (e.g. sodium concentration, urinary specific urine gravity) in this group of patients.
The aims of the study were to assess the level of serum copeptin in children with PMNE and to look for a relation between copeptin and selected clinical and biochemical parameters in these children.
Material and methods
Eligibility criteria
The children recruited for the trial fulfilled all of the following inclusion criteria: clinical diagnosis of PMNE, age above 5 to 15 years, normal creatinine level, and normal ultrasonography image of kidneys and urinary tract.
Exclusion criteria were as follows: NMNE, and states that could influence copeptin level according to the literature chronic diseases: hypertension, chronic kidney disease, obesity (BMI > 85th percentile), metabolic syndrome, congenital heart defects, diabetes, hematologic disease, and acute conditions such as infections, dehydration, overhydration, or deep stress.
All children with PMNE were evaluated to fulfill the inclusion and exclusion criteria. The following clinical parameters were evaluated in the analyzed children: age, sex, height [cm], frequency of bed-wetting (based on a bedwetting diary) and bladder capacity (based on a micturition diary). In all the patients abdominal ultrasound (ALOKA Prosound Alpha 6, Hitachi ALOKA, Japan) was performed with assessment of kidneys and the urinary tract.
The percentage ratio of the obtained urinary bladder capacity (BC) to the expected bladder capacity (EBC) was calculated (BC/EBC ratio). EBC was calculated according to the Koff formula: (age [years] + 1) × 30 [ml] [19]. Increased bladder capacity was defined as BC/EBC ratio ≥ 150%. According to the BC/EBC ratio the study group was divided into two subgroups: patients with normal and increased bladder capacity (supposed mechanism of nocturnal enuresis – nocturnal polyuria) and decreased bladder capacity (supposed mechanism of nocturnal enuresis – nocturnal detrusor overactivity).
In all the children in the morning (8-10 a.m.) a blood sample was taken. In the blood sample copeptin was determined by ELISA (enzyme-linked immunosorbent assay) using the Copeptin Human ELISA Kit EK-065-32CE (Phoenix Pharmaceuticals, Inc., Burlingame, California, USA) [pg/ml]. In addition, in all the children the following tests were evaluated: serum creatinine (mg/dl), glomerular filtration rate (GFR) according to the Schwartz formula (ml/min/1.73 m2) [20], sodium (mmol/l) and potassium (mmol/l), complete blood count and urinalysis with specific urine gravity. In all the patients a water deprivation test was performed. For the test patients were admitted to hospital and fluid intake was stopped at 6 p.m. Every morning a urine sample was assessed for specific gravity using hydrometry. Water deprivation was discontinued when one of the following criteria was met: an urine reached specific gravity 1025 or higher, a weight loss of more than 5% from baseline or symptoms of water deprivation were intolerable for the patient.
None of the studied children with PMNE were treated during the diagnostic tests. After the tests were completed, the treatment was implemented according to International Children’s Continence Society guidelines [2] using the following methods: evening fluid restriction, motivational training, bed-wetting alert, desmopressin, or anticholinergic agents. In refractory cases treatment was individualized with implementation of combined therapy.
Twenty healthy children were included in the control group. The following parameters were evaluated in these children: serum copeptin, creatinine, sodium, potassium levels, hematocrit from complete blood count, and urine specific gravity.
The research project was approved by the local Ethics Committee (approval No. KB/102/2017). All procedures were performed in accordance with the Declaration of Helsinki on the treatment of human subjects. Informed consent was obtained from all participants’ representatives included in the study.
Statistical analyses
Statistical analyses were performed using Dell Statistica 13.0. All the results were presented as mean values ± standard deviation. Normality of the data was tested using the Shapiro-Wilk test. Differences in normally distributed variables were tested using the Student t-test and in variables with other than normal distribution using the Mann-Whitney U test. Differences in the frequency of analyzed variables between the two groups were analyzed using the chi-square test or the Fisher exact test when appropriate. P < 0.05 was considered statistically significant.
Results
Clinical characteristics of the study group are presented in Table 1. More than two thirds of the studied children were boys. Mean age was about eight and did not differ significantly between boys and girls (8.64 ±2.33 vs. 7.41 ±2.50 [years], p = 0.062). All the children had normal renal function. The frequency of bed-wetting varied from 1 to 7 nights per week; 12 (48.0%) children had every night wetting. Normal bladder capacity was found in 11 (44.0%) children, increased in 2 (8.0%), and decreased in the remaining 12 (48.0%) patients.
Table 1
Clinical characteristics of the studied children
Comparison of copeptin level and clinical and biochemical parameters between the study and control group is presented in Table 2. Children from study and control groups did not differ in terms of sex or age. Serum copeptin level did not differ significantly between the groups. Also the groups did not differ in hematocrit, serum creatinine or sodium or in specific urine gravity. Serum potassium level was normal in all the subjects but mean serum potassium was significantly higher in the study group compared to the control group.
Table 2
Comparison of copeptin and clinical and biochemical parameters between the study and control groups
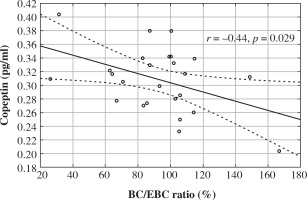
In the study group we found a tendency towards positive correlations between serum copeptin level and age (r = 0.39, p = 0.056), hemoglobin (r = 0.40, p = 0.060). No significant correlations between copeptin and hematocrit (r = 0.28, p = 0.200), GFR (r = 0.22, p = 0.288), serum sodium (r = 0.12, p = 0.563), and potassium levels (r = 0.34, p = 0.090), morning specific urine gravity (r = 0.14, p = 0.525) and specific urine gravity in the water deprivation test (r = 0.05, p = 0.830). Also serum copeptin level did not correlate with frequency of bed-wetting (r = 0.10, p = 0.639). Serum copeptin correlated only negatively with BC/EBC ratio (r = –0.44, r = 0.029) (Fig. 1).
Fig. 1
The relation between serum copeptin level and bladder capacity/expected bladder capacity ratio in children with primary monosymptomatic nocturnal enuresis
We found no differences in copeptin level between boys and girls – 0.317 ±0.042 vs. 0.289 ±0.053 [pg/ml], p = 0.165; between children with low morning specific urine gravity (defined as s.g. ≤ 1010) and normal morning specific urine gravity (s.g. > 1010) – 0.306 ±0.051 vs. 0.305 ±0.045 [pg/ml], p = 0.970; also no differences between children with low specific urine gravity in the water deprivation test (defined as s.g. < 1025) and normal water deprivation test specific urine gravity (s.g. ≥ 1025) – 0.311 ±0.066 vs. 0.300 ±0.029 [pg/ml], p = 0.606; and no differences between those with small bladder capacity and normal bladder capacity – 0.319 ±0.041 vs. 0.298 ±0.051 (pg/ml), p = 0.274.
The comparison of copeptin and clinical and biochemical parameters between 13 patients with normal or increased bladder capacity and the control group is presented in Table 3. We found no differences in copeptin concentration and other analyzed clinical and biochemical parameters between the groups. In the subgroup of 13 children with normal/increased bladder capacity (subgroup with suspected nocturnal polyuria) we found no significant correlations between serum copeptin level and age (r = 0.42, p = 0.150), hemoglobin (r = 0.35, p = 0.240), hematocrit (r = 0.20, p = 0.505), GFR (r = 0.33, p = 0.267), serum sodium (r = 0.17, p = 0.583), morning specific urine gravity (r = –0.06, p = 0.854) and specific urine gravity in water deprivation test (r = 0.18, p = 0.556), or with frequency of bed-wetting (r = 0.28, p = 0.350). We found only trends towards correlations between serum copeptin and potassium level (r = 0.50, p = 0.080) and with BC/EBC ratio (r = –0.48, p = 0.095).
Table 3
Comparison of copeptin and clinical and biochemical parameters between thirteen patients with normal/increased bladder capacity (nocturnal polyuria) and the control group
No significant correlations were found between serum copeptin level and analyzed clinical and biochemical parameters in the control group.
Discussion
In our study we analyzed serum copeptin levels in children with PMNE in relation to clinical and biochemical parameters. We did not find any differences in terms of serum copeptin level between children with nocturnal enuresis and healthy children. In children with PMNE we found no relation between serum copeptin level and sex, kidney function, sodium, or urinary specific gravity. We found only a negative relation between copeptin and bladder capacity and trends towards positive relations between copeptin and age, as well as hemoglobin. Interestingly, in the subgroup of children with normal bladder capacity there was a trend towards a positive correlation between serum copeptin and potassium levels.
Theoretically, in children with PMNE with disturbed nocturnal antidiuretic hormone secretion morning copeptin levels should be lower compared to healthy peers. In our small group of patients we did not confirm that copeptin level could be a reliable marker of PMNE. On the other hand, we found a significant negative correlation between bladder capacity (expressed as BC/EBC ratio) and copeptin level. This relation seems to confirm a different mechanism of nocturnal enuresis depending on bladder volume. We assume that in children with normal or high bladder capacity copeptin levels are lower as a consequence of a defect of ADH secretion and in those with small bladder capacity are normal, as these patients have a different mechanism of bed-wetting. Notably, the subgroup of patients with nocturnal enuresis and normal/increased bladder capacity did not differ in copeptin level from the subgroup of patients with decreased bladder capacity (nocturnal detrusor overactivity) and from the control group. This could be, in our opinion, a consequence of the small patient group. Also, the normal sodium level and no difference in copeptin level could suggest other pathogenetic factors of nocturnal enuresis in our study group. It should be emphasized that desmopressin is no “miracle drug” for bed-wetting and 20 to 60% of children with monosymptomatic enuresis are desmopressin-resistant [3, 18]. The results of the study by Hara suggest that a high day-to-night copeptin ratio, suggesting a night ADH release defect, might be a predictor of clinical response to desmopressin [18].
Comparable serum copeptin concentrations in children with PMNE and in healthy peers are in opposition to the results of Nalbantogˇlu et al. [17]. These authors found significantly lower levels of copeptin in children with bed-wetting than in the control group. The patients in Nalbantogˇlu’s group were slightly older (9.70 ±2.04), and copeptin was measured in plasma, not in serum, and using a different method (coated tube immunoluminometric assays), which could have influenced the results. Interestingly, the level of copeptin in Nalbantogˇlu’s study was approximately 10 times higher compared to our results. This striking difference raises a question about the variability and reproducibility of different assays evaluating copeptin level. The ELISA assays used in our study may detect fragments of proteins as well as conglomerates of particles. Further studies are necessary before copeptin can become a reliable marker of PMNE in everyday clinical practice.
Male sex is more common among patients with PMNE, as was found in our sample [1-3]. In our study group there were no differences in copeptin level between boys and girls with PMNE. Tenderenda-Banasiuk [15] and Enhörning [10] found higher copeptin levels in boys compared to girls. In our study group copeptin level did not differ between the genders. A similar lack of difference between genders was presented in studies by Skrzypczyk [14] in children with chronic kidney disease and by Przybyłowski [21] in people after heart transplantation. Rothermel et al. found significantly higher copeptin levels in boys compared to girls only after sexual maturation, without differences in the prepubertal period [22]. In animal studies antidiuretic hormone secretion was similar for both sexes with significant differences in the rate of hormone degradation [23]; we cannot exclude a similar mechanism in humans. Depletion of intravascular volume is a key stimulus for antidiuretic hormone secretion. Vasopressin binding to V2 receptors stimulates free water reuptake in collecting ducts, thus increasing intravascular volume and simultaneously leading to a drop in serum sodium level, as seen in patients with the syndrome of inappropriate antidiuretic hormone (SIADH) release [24]. We did not find any significant relation between serum copeptin level and serum sodium. Most children before blood sampling have already been treated non-pharmacologically with evening fluid restriction, which could interfere with this relation. On the other hand, we found a tendency towards a positive correlation between copeptin and hemoglobin. Similarly, Skrzypczyk found a linear relation between copeptin and hemoglobin level in 38 children with chronic kidney disease (CKD) suggesting that copeptin could be a marker of hydration in these patients [14]. In the study by Przybyłowski copeptin was not related to hemoglobin in patients after heart transplantation [21]. In the study of 2113 adult healthy people (median value of age 36.7 years) similar linear relations between hemoglobin and copeptin were revealed [25]. Thus, we think that copeptin could be a sensitive marker of intravascular volume in different pediatric populations.
All patients from both study and control group had normal serum potassium level. Nevertheless, interestingly serum potassium level was higher in patients with enuresis. In addition, in a subgroup of 13 patients with normal bladder capacity we found a trend towards positive correlation between serum copeptin and potassium level. It is well known that hypokalemia leads to development of polyuria, probably in the mechanism of downregulation of aquaporin-2 water channel expression in kidneys [26]. Rittig et al. found that children with nocturnal enuresis were characterized by higher nocturnal kaliuresis compared to healthy peers [27]. So the higher serum potassium level in children with PMNE is surprising and requires further studies on the influence of this ion on the pathogenesis and clinical course of bed-wetting. Also, the relation between potassium and copeptin is unclear. A drop in intravascular volume leads to release of ADH (and copeptin) but also activation of the renin-angiotensin-aldosterone system and increased urinary potassium loss. Accordingly, in the study by Akinladel et al., adult patients with schizophrenia were characterized by higher copeptin and lower potassium level compared to the control group [28]. On the other hand, Riphagen in a study of 25 adult prehypertensive individuals on a lowsodium diet found that supplementation of potassium (72 mmol/day) led to a decrease in 24-hour blood pressure and atrial natriuretic peptide (ANP) but also caused increases in renin, aldosterone and plasma copeptin. The authors concluded that a rise in copeptin (and vasopressin) could be a compensatory mechanism against potassium-induced blood pressure decrease [29]. In contrast, van Gastel et al. found in 6801 patients from the PREVEND (prevention of renal and vascular endstage disease) study that higher copeptin levels were determined by low urinary potassium loss (suggesting low potassium intake) [30].
Clear limitations of our study are the small sample size and cross-sectional character of the study. The sample size could have influenced some of the results – high correlation coefficients in many cases (e.g. for the relation between hemoglobin, age, potassium and copeptin) suggest that these dependences could have reached clinical significance in larger patient groups. Also, we did not measure night urine production in patients from the study group. Thus, the division of patients into subgroups with different mechanisms of nocturnal enuresis (night polyuria and night overactive bladder) was based solely on the BC/EBC ratio. In addition, most patients before blood sampling had already been treated with fluid restriction, which could have influenced the copeptin level and its relation with sodium and specific urine gravity. Moreover, we did not perform a second analysis of these patients during the treatment and could not analyze the influence of e.g. desmopressin on serum copeptin level.
Conclusions
Serum copeptin level seems not to be a reliable marker of primary monosymptomatic nocturnal enuresis.
Low serum copeptin levels in children with PMNE may suggest a defect in antidiuretic hormone release and normal bladder capacity.
Copeptin may be a marker of hydration status in children with PMNE.
The relation between potassium and copeptin level and the clinical significance of its relation require further studies.


