Introduction
High-dose chemotherapy with autologous stem cell transplant (ASCT) is a widely used therapy in the treatment of haematopoietic neoplasms. However, ASCT is associated with profound deficiencies in the function of the immune system. The proportion of ASCT recipients who go on to develop severe infections and associated complications varies, but infections are still a significant cause of mortality and morbidity. Age, general health, co-morbidities, and the number of previous treatment regimens are some factors known to influence the risk of such complications. In a study by Weaver et al., the mortality rate due to infections after high-dose chemotherapy and ASCT was 1.5% [4]. However, new prognostic markers are still being explored, the knowledge of which would allow us to determine the risk of severe complications in individual patients before the start of high-dose chemotherapy.
Toll-like receptors (TLRs) are a group of proteins that are actively involved in the function of the immune system. Most TLRs are expressed on the surface of cells, although TLR3 and TLRs 7-9 are intracellular [1]. TLRs are found on haematopoietic cells, such as B lymphocytes, T lymphocytes, monocytes, macrophages, dendritic cells, and NK cells, as well as on non-haematopoietic cells, including vascular endothelial cells, gastrointestinal and respiratory epithelial cells, adipocytes, cardiomyocytes, and fibroblasts. Their diverse expression means TLRs come into contact with many pathogens attempting to penetrate the body. Their recognition of these pathogens can stimulate the immune system.
One of the mechanisms of TLR function is the activation of the NF-κB transcription factor. The TLRs that are expressed on macrophages and dendritic cells initiate an innate immune response, including the activation of phagocytosis processes and the production of inflammatory chemokines and cytokines such as interleukin (IL)-12, IL-6 and type I interferon (type I IFN). TLRs are often described as a bridge between an innate immune response and an acquired immune response [3].
This study aimed to evaluate the expression of Toll-like receptor genes and their correlation with the incidence of infection after ASCT in patients with haematological malignancies.
Material and methods
Study participants
The expression of Toll-like receptor genes (TLR2, TLR4, and TLR9) was measured in 60 patients undergoing ASCT. The median age of the patients was 54 years (range: 25-65 years). ASCT was performed due to multiple myeloma (MM) in 20 patients, for non-Hodgkin’s lymphoma (nHL) in another 20 patients, and Hodgkin’s lymphoma (HL) in 20 patients. The healthy control group include 10 volunteers. The median age of healthy controls was 51 years (range: 26-64 years).
Peripheral blood samples were taken for analysis before the start of high-dose chemotherapy, before ASCT, and at the time of haematopoietic regeneration after stem cell transplantation (median: 14 days; range: 10-16 days after ASCT). In patients with MM, high doses of melphalan were administered (200 mg/m2 or 140 mg/m2, depending on the patients’ age and general health) during the conditioning regimen before ASCT. Patients with nHL and HL received high-dose chemotherapy according to the BEAM protocol (carmustine, etoposide, cytosine arabinoside, and melphalan).
A median of 3.38 × 106 autologous CD34-positive cells per kilogram of body weight were transplanted. The median duration of neutropenia was 10 days in patients with MM (range: 9-15 days) and 12 days in patients with nHL and HL (range: 11-17 days). The median granulocyte regeneration time (defined as > 500 neutrophils/µl) was 14 days in the entire study population (range: 10-16 days). Febrile neutropenia was observed in 30 patients (8 patients with MM, 12 patients with nHL and 10 patients with HL). Microbiologically confirmed infection was found in 24 cases (4 patients with MM and 20 patients with nHL and HL). The most frequently isolated pathogen was Klebsiella pneumoniae (10 cases). Infection was confirmed in stool culture in 8 patients, and in blood culture in 2 patients. In 8 patients, Enterococcus faecalis was isolated from the faeces. For prophylaxis of infections, ciprofloxacin, fluconazole, aciclovir and trimethoprim were administered to patients before ASCT. The median response time to antimicrobial treatment in the patients with infection was 4 days (range: 2-10 days).
All patients and healthy volunteers signed informed consent forms in order to participate in the study. The study was approved by the Bioethics Committee of the Medical University of Wroclaw. The clinical data of the patients are presented in Table 1.
Table 1
Patient clinical data
Real-time PCR
The relative expression of Toll-like receptors TLR2, TLR4, and TLR9 was assessed by real-time polymerase chain reaction (PCR) using TaqMan Assays (Life Technologies/Thermo Fisher). Beta glucuronidase (GUSB) served as an endogenous control. The reaction was carried out on a 7500 Real-Time PCR instrument (Life Technologies) using Gene Expression Master Mix (Life Technologies/Thermo Fisher). The comparative CT method was used to compare the expression of patients with healthy controls.
Statistical analysis was conducted using STATISTICA 12 software (StatSoft, Poland). For quantitative variables, arithmetic means (X) and standard deviations (SD) of the estimated parameters were calculated for the study groups. The distribution of variables was tested using Lilliefors and Shapiro-Wilk W tests. In cases of independent quantitative variables with normal distribution, a t-test for independent variables was used. In cases of variables with a non-normal distribution, the t-test for dependent variables was applied to the quantitative variables of the normal distribution. In cases of quantitative dependent variables with non-normal distribution, Wilcoxon’s pair sequence test was applied. In order to define the relationships between the studied variables, correlation analysis was performed. Results at the level of p < 0.01 were considered statistically significant.
Results
In comparison to the control group, expression of TLR4 was decreased in patients (ΔCt TLR4 22.21 ±0.32 vs. 11.91 ±70.22, p < 0.01), while TLR2 gene expression was higher in patients than in healthy individuals (ΔCt TLR2 6.46 ±9.58 vs. 0.98 ±0.43, p < 0.05). TLR9 gene expression was higher in the control group than in haematological patients (ΔCt TLR9 13.65 ±3.29 vs. 3.35 ±1.93, p < 0.05).
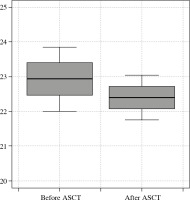
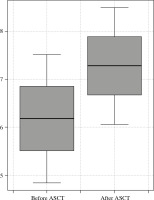
The expression of TLR4 was statistically significantly higher in patients who had not yet had transplant-related, high-dose chemotherapy than in those who had undergone ASCT (Ct TLR4 22.93 ±0.93 vs. 22.4 ±0.64). The expression of TLR9 was significantly lower before ASCT than in patients who had undergone ASCT (dCt TLR9 6.18 ±1.34 vs. 7.28 ±1.22, respectively, p < 0.01). The results are presented in Table 2 and in Figures 1 and 2.
Table 2
Comparison of TLR values before and after aut ologous stem cell transplantation (ASCT)
The expression of TLR4 and TLR9 measured before the start of high-dose chemotherapy was significantly lower in patients who presented symptoms of infection in the post-transplant period (Ct TLR4 22.73 ±1.07 vs. 23.07 ±0.81 and Ct TLR9 28.5 ±1.24 vs. 26.71 ±1.18. respectively, p < 0.01). In patients with a clinically significant bacterial infection as a post-transplant infectious complication, significantly higher TLR9 expression was observed compared to patients without any signs of an infection (Ct TLR9 116.34 ±123.67 vs. 105.83 ±98.23). Such a relationship was not observed for TLR4 and TLR2 expression. TLR9 expression and neutrophil cell growth after stem cell transplantation were positively correlated (r = 0.4075, p = 0.023). We detected no significant differences in TLR expression or infection rate between patients with MM, nHL, and HL. TLR expression was similar in patients with infection and MM, nHL and HL. The results are presented in Table 3.
Table 3
Comparison of TLR values before autologous stem cell transplantation (ASCT) in patients with infection and without infection
Discussion
Infections are a common complication following ASCT. Depending on the period of bone marrow aplasia in which they appear, their course can be severe and can even lead to the death of the patient. Infections with Gram-positive and Gram-negative bacteria occur in about 20% of patients after autotransplantation, including bacteraemia in 7-8% of patients. Viral infections are observed in about 10-11% of patients after ASCT. Invasive mycoses are quite rare [5]. The mortality associated with severe infections after autotransplantation is estimated at around 2-4% of patients [5-7]. Therefore, new factors and prognostic markers that enable the identification of patients with a higher risk of infection are of clinical importance.
The role of TLRs in the post-transplantation course remains unclear. The mechanisms of TLR action in solid organ transplantation have been much more thoroughly examined. Studies have shown that TLR11 plays a crucial role in the development of infections after renal transplantation. The most frequent infection-related complications are asymptomatic bacteriuria, cystitis, and pyelonephritis. These infections increase the risk of early mortality following transplantation [8].
TLR11 acts as a physiological barrier that protects the body against the penetration of uropathogens [8, 9]. Studies on mice have shown that TLR11 is expressed in liver, kidney, bladder, and vascular endothelial cells, and has an affinity with a uropathogenic E. coli strain, thereby affecting local immunity in the urogenital tract [10]. It has also been revealed that higher TLR11 expression strengthens immunity against Toxoplasma gondii [11]. Finally, Shi et al. reported that invasive Salmonella infection occurred in mice with diminished TLR11 levels [12].
Innate immunity, of which TLRs are a critical element, plays an essential protective role against pathogens after a liver transplant. This protective role is particularly important when it comes to infections of the hepatitis C virus (HCV). Recurrence of HCV infection after liver transplantation is common [13-15], and HCV reactivation causes aggressive liver fibrosis and graft rejection in approximately 30% of HCV-infected patients [16, 17]. Eid et al. demonstrated that the Arg753Gln TLR2 polymorphism correlates with progressive cirrhosis of the transplanted liver, graft rejection and patient death after liver transplantation due to HCV-dependent cirrhosis [18].
The role of TLRs in infectious complications after stem cell transplantation has not yet been clearly defined. In a prospective analysis, Skert et al. assessed the expression of TLRs in T lymphocytes and monocytes in a population of 35 patients after bone marrow stem cell transplantation and its correlation with infectious complications. The authors found that TLR9 expression in T lymphocytes correlates negatively with bacterial infections. A correlation was also observed between TLR7 expression and fungal infections in this patient population [19].
In this study, we found that the expression of TLR4 and TLR9 before the start of the transplant procedure was statistically significantly lower in patients who had severe infectious complications in the later period. Additionally, TLR9 expression significantly increased in patients with an active infection compared to patients without infection after transplantation. The physiological function of TLR4 is to stimulate the production of tumour necrosis factor α (TNF-α), interleukin (IL)-1, and IL-12 after binding with the pathogen-associated molecular pattern (PAMP) ligand of the pathogenic agent. The immune system is only activated when TLR4 acts in conjunction with the antigens CD14 and CD11b/CD18 [9, 20]. TLR9 acts by stimulating IL-12, monocytes, macrophages and NK cells. TLR9 is often activated by bacterial DNA, which results in the creation of a natural defence mechanism against bacteria [20, 21]. Consistently, experiments have shown that blocking the gene for the TLR9 receptor results in inhibition of activity against bacterial proteins [23, 24]. Lower expression of TLRs causes reduced synthesis of cytokines and chemokines, which leads to a generalised reduced immune response [22].
We also observed a positive correlation between TLR9 and the number of neutrophils during the period of bone marrow regeneration, which may indicate a role of TLRs in the reconstitution of the immune system following transplantation.
Conclusions
The role of TLRs in the immunological processes which determine immunity against infectious agents is crucial. As demonstrated by the results of our research, and supported by previous publications, lower expression of individual TLRs (especially TLR4 and TLR9) may correlate with a higher risk of infections, especially in immuno-incompetent patients after stem cell transplantation.