Introduction
Diseases of the scalp related to hair loss are a common problem in the population, significantly affecting the quality of life. There are two types of alopecia: scarring and non-scarring. Among patients suffering from scarring hair loss, one of the largest group comprises patients with lichen planopilaris (LPP), which is a cause of alopecia in about 1.25% of patients. Frontal fibrosing alopecia (FFA) is currently considered a clinical variant of LPP sharing some similar histopathologic findings [1–4]. A common cause of non-scarring alopecia is alopecia areata (AA), which estimated prevalence is about 0.1 to 0.2% in the general population [5].
LPP is a primary lymphocytic scarring alopecia, characterized by a chronic lymphocytic infiltrate that destroys hair follicles. Various studies have indicated that LPP seems to be a hair-specific autoimmune disease, where activated T lymphocytes are directed against unknown follicular antigen. Both primary and acquired immune responses are involved in the pathogenesis of this disease. However, the pathogenesis of LPP remains not completely elucidated and this disease still represents a therapeutic challenge [6–10].
S100 proteins constitute a family of calcium-binding, low molecular weight (9–13 kDa) molecules that are engaged in the control of a wide range of intra- and extracellular functions [11]. Some of S100 proteins may act as damage-associated molecular pattern (DAMP) – alarmins with the ability to induce proinflammatory responses and provoking autoimmune diseases. Recently, it has been suggested that some of these proteins may play an important role in the pathogenesis of chronic immune-mediated inflammatory skin diseases [12–14]. S100A7 (psoriasin), one of the members of the S100 protein family, is encoded by genes located in epidermal differentiation complex (EDC) on chromosome 1q21 and is comprised of two calcium-binding sites of the helix-loop-helix (‘EF-hand type’) conformation. S100A7 is an antimicrobial protein (AMP) with proinflammatory properties. S100A7 was first identified as overexpressed by keratinocytes in psoriatic lesions, hence it was named “psoriasin” [14–17]. Apart from its role in psoriasis, an overexpression of S100A7 was also found in other inflammatory skin diseases with epidermal abnormalities such as lichen planus, Darier’s disease and hidradenitis suppurativa [11, 18, 19]. S100A7 has been shown to act as a chemoattractant for leukocytes. It stimulates skin cells such as keratinocytes for increased production of proinflammatory mediators combining innate and adaptive immune mechanisms [17, 20]. S100A4 (also known as metastasin, calvasculin or fibroblast-specific protein 1) is another member of the S100 protein family. It was first described to be involved in induction and promotion of tumour metastasis. However, it has also been proven that apart from its function in cancer progression and metastasis, S100A4 is implicated in the pathophysiology of fibrotic, autoimmune inflammatory disorders. Recent studies have indicated the involvement of S100A4 in the pathogenesis of several diseases such as systemic sclerosis, hypertrophic scars or psoriasis. Therefore, S100A4 can serve as a competent biomarker for diagnosis and monitoring of fibrotic diseases [21–26].
IL-17 is an important proinflammatory cytokine whose role in the pathogenesis of various inflammatory diseases has been proven, it is now also known to be implicated in the development of various autoimmune skin diseases. The main production of IL-17 is carried out by T-helper (Th) 17 cells, however it is also produced by neutrophils, natural killer cells, mast cells and T regulatory (Treg) cells. IL-17 evokes cellular reactions in keratinocytes, fibroblasts, neutrophils and endothelial cells. It is characterized by a proinflammatory activity with the ability to induce the expression of other proinflammatory cytokines and chemokines [5, 27–29].
Aim
The objective of this study was to evaluate the expression of S100A7, S100A4 and IL-17 in lesional skin obtained from patients suffering from LPP as compared to lesional skin taken from patients with AA and to normal skin of healthy controls.
Material and methods
Study group
The study group consisted of 23 individuals with clinically suspected and histologically confirmed LPP. Among those subjects, 14 patients had histologically confirmed classical LPP, and 9 patients had histologically confirmed FFA. The duration of the disease was several months (mean: 10.1 ±22.3 months). The biopsy specimens were obtained from LPP lesional skin. Biopsy specimens obtained from individuals with clinically and histologically confirmed AA (n = 11) served as a comparator. The patients did not receive any topical treatment or any systemic treatment at least 4 weeks before biopsy. Exclusion criteria included other skin diseases, neoplasms, other chronic immune-mediated inflammatory diseases, chronic or acute uncontrolled conditions. Biopsy specimens of normal skin taken from healthy, age- and gender-matched individuals during plastic surgery procedures served as controls (n = 14). The characteristics of the study group are presented in Table 1.
Tissue expressions of S100A7, S100A4, IL-17
The tissue expressions of S100A7, S100A4 and IL-17 were assessed with an immunohistochemical method. The immunohistochemical reactions was performed on 4 μm-formalin-fixed and paraffin-embedded (FFPE) biopsy specimens using mouse anti-human psoriasin (S100A7) monoclonal antibodies (catalogue number ab13680; Abcam, UK), mouse anti-human S100A4 monoclonal antibody (catalogue number ab218512; Abcam, UK), mouse anti-human IL-17 polyclonal antibodies (catalogue number PA1-84183; Invitrogen, USA) according to the manufacturer’s protocol, with positive and negative control stainings. Detection was performed on the platform Dako using the Dako RDS AP kit (Dako Real Detection System Alkaline Phosphatase Real Rabbit/Mouse, catalogue number K5005). The preparations were assessed using Zeiss Axio Imager A2 light microscope with video track and Zeiss AxioVision software. The expression was considered as positive when the membrane or cell cytoplasmic immunoreactivity was observed. Positively stained cells were counted in 10 fields of view for each specimen preparation at 200× magnification, and the mean value for each preparation was calculated.
Statistical analysis
The obtained results were subjected to statistical analysis. The Shapiro-Wilk normality test and Kolmogorov-Smirnov normality test were used to determine the normality of variable distribution. The differences between the groups were determined using Student’s t-test (for continuous variables showing normal distribution) or the Mann-Whitney U test (for continuous variables not showing normal distribution). The χ2 test (with the Yates correction test) and the Fisher exact test were used to compare categorical variables. P-value < 0.05 was considered statistically significant. All statistical calculations were made using Graph Pad Prism version 5.01 software.
Results
Expression of S100A7
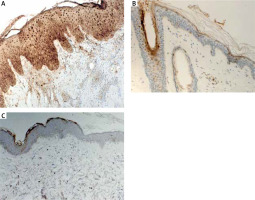
The quantitative evaluation of S100A7 expression in histological sections was presented as the mean number of positively stained cells in the field of view ± SD (Table 2). The number of cells showing S100A7 expression was significantly higher in LPP lesional skin as compared to AA lesional skin (p = 0.0002) and to normal skin of healthy controls (p < 0.0001) (Table 2). The expression of S100A7 in patients with AA and normal skin was relatively low, present in upper layers of epidermis and in hair follicles (Figure 1). The number of cells showing S100A7 expression was not significantly different in AA lesional skin and normal skin (p > 0.05). In LPP lesional skin, S100A7 was expressed by keratinocytes in epidermis and in hair follicles. S100A7 was also expressed by cells within the inflammatory infiltrate, mainly lymphocytes and other leukocytes (Figure 1). The number of cells showing S100A7 expression was not significantly different in classical LPP (25.13 ±6.82) and its variant FFA (24.0 ±5.13) (p > 0.05). Representative immunohistochemical stainings of S100A7 in LPP lesional skin, AA lesional skin and in normal skin of healthy controls is presented in Figure 1.
Table 2
The quantitative evaluation of S100A7, S100A4, IL-17 expression in LPP lesional skin, AA lesional skin and normal skin of healthy controls (values expressed as the mean number of positively stained cells ± SD)
Expression of IL-17
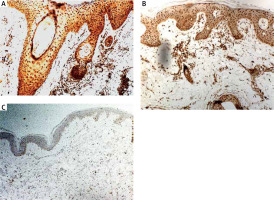
The quantitative evaluation of IL-17 expression in histological sections was presented as the mean number of positively stained cells in the field of view ± SD (Table 2). The number of cells showing IL-17 expression was significantly higher in LPP lesional skin as compared to normal skin of healthy controls (p < 0.0001). The number of cells showing IL-17 expression was not significantly different in classical LPP (91.92 ±13.9) and its variant FFA (88.36 ±6.8) (p > 0.05). In LPP lesional skin, IL-17 was expressed in keratinocytes in epidermis and in hair follicles as well as by cells within the inflammatory infiltrate, mainly lymphocytes and other leukocytes (Figure 1). The number of cells showing expression of IL-17 in the normal skin was relatively low. The number of cells showing IL-17 expression was significantly higher in AA lesional skin as compared to normal skin of healthy controls (p < 0.0001). Representative immunohistochemical stainings of IL-17 in LPP lesional skin, AA lesional skin and in normal skin are presented in Figure 2.
Expression of S100A4
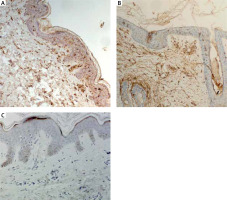
The quantitative evaluation of S100A4 expression in biopsy specimens was presented as the mean number of positively stained cells in the field of view ± SD (Table 2). The number of cells showing S100A4 expression was not significantly different in LPP lesional skin, AA lesional skin and in normal skin from healthy controls. In lesional skin of LPP, the number of S100A4 expressing cells was relatively low. Representative immunohistochemical stainings of S100A4 in LPP lesional skin, AA lesional skin and in normal skin of healthy controls are presented in Figure 3.
Discussion
Although numerous studies indicate a fairly high prevalence of LPP in the population, little is known about its pathogenesis and the exact cause of this disease remains to be elucidated [30, 31]. LPP seems to be one of T-cell-mediated autoimmune diseases, with the involvement of innate and acquired immune responses [6, 8, 9]. Histologically LPP and its variant FFA are characterized by an inflammatory infiltrate composed predominantly of lymphocytes, located between the follicular epithelium and the dermis, with the involvement of the isthmus and infundibulum of the hair follicles and reduced number of hair follicles. Other histopathological findings are hyperkeratosis of interfollicular epidermis, follicular plugging, perifollicular fibrosis in the external root sheath [32–34]. The alteration of scalp microbiota has been recently reported in LPP. Skin microbiota by regulation of the expression of antimicrobial proteins on keratinocytes and other cells may influence the immune response [35].
S100A7 is a multifunctional, antimicrobial protein with proinflammatory properties. It was first identified as overexpressed in psoriatic skin lesions. In normal skin, expression of S100A7 is low and restrained to the hair follicles and granular/cornified layers of the interfollicular epidermis. In psoriatic lesions, S100A7 is overexpressed by keratinocytes and infiltrating leukocytes [11, 14–17, 36]. In the current study, we investigated the expression of S100A7 using an immunohistochemical assay in biopsy specimens from lesional skin obtained from individuals with histologically confirmed LPP. To the best of our knowledge, this is the first study investigating the expression of S100A7 in LPP. We found a significantly higher number of cells showing S100A7 expression in lesional skin taken from patients with LPP as compared to lesional skin taken from patients with AA and to normal skin of healthy controls. S100A7 was overexpressed by keratinocytes in interfollicular epidermis and in hair follicles. S100A7 was also present in the dermal inflammatory infiltrate. It has been shown that some proinflammatory factors, including bacterial products or proinflammatory cytokines such as TNF-α, IL-17 and IL-22 are potent to induce overexpression of S100A7 in the skin [14, 15, 20, 37]. The role of the dysbiosis of skin microbiota on the modulation of the innate and adaptative immune response and the development of autoimmune diseases is suggested [14, 35]. S100A7, overexpressed in the skin, acts as DAMP – alarmin and primes keratinocytes for enhanced production of proinflammatory cytokines and chemokines, including TNF-α, IL-6, IL-8 [20]. At the same time S100A7 has the ability to function as a chemotactic agent for leucocytes and to attract lymphocytes and neutrophils to the site of inflammation [17]. S100A7-dependent signalling is mediated through the multiligand receptor for advanced glycated end products (RAGE). S100A7-RAGE interactions activate multiple intracellular signalling pathways, including signal transducer and activator of transcription 3 (STAT3), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and activator protein 1 (AP-1) resulting in increased expressions of proinflammatory cytokines and cellular adhesion molecules [14, 17]. The results of our study may point toward the involvement of S100A7 in the pathogenesis of LPP.
S100A4 has been shown to be involved in the pathophysiology of autoimmune and fibrotic disorders. Various studies have pointed out the involvement of S100A4 in pathogenesis of several diseases [22, 23, 25]. The immunohistochemical examination of psoriasis lesional skin biopsies revealed significant upregulation of S100A4 in the upper dermal part of lesional skin [25]. Also increased expression of 100A4 protein was found in the lesional skin of patients with systemic sclerosis [24]. LPP is a cause of scarring hair loss [3]. To the best of our knowledge, in the current study we investigated, for the first time, expression of S100A4 in LPP lesional skin samples. We did not find any significant differences between the expression of S100A4 in LPP lesional skin as compared both to AA lesional skin and normal skin. Further research is required to assess the involvement of S100A4 in the pathogenesis of LPP.
IL-17 is a proinflammatory cytokine. It is involved in the defence mechanism against pathogens through production of various chemokines, cytokines and antimicrobial proteins but also promotes inflammation. IL-17 has the ability to attract neutrophils and macrophages to the inflammation site thus triggering an excessive immune response and leading to the development of inflammatory and autoimmune diseases [38]. Żychowska et al. have indicated a significantly higher number of cells showing IL-17 expression in patients with cutaneous lichen planus compared to normal skin [28]. The pathophysiology of lichen planopilaris is not fully understood but is probably related to the cause of lichen planus [39]. In our study, we showed increased expression of IL-17 in LPP lesional skin as compared to AA lesional skin and normal skin. IL-17 was overexpressed in the inflammatory infiltrate and in the epidermis. IL-17 may act on keratinocytes and activate these cells for increased production of proinflammatory and chemotactic factors, enhancing an inflammatory loop [27, 29]. Our results are consistent with previous, recently published studies. Shahidi Dadras et al. have shown that the average number of IL-17+ cells was significantly higher in LPP compared to controls [40]. Another study of Miteva et al. has revealed increased expression of IL-17 in the perifollicular fibrosis of the affected follicles in LPP when compared to controls [41]. These results highlight the possible role of IL-17 in the pathogenesis of LPP. AA is thought to be a disease of a multifactorial aetiology, where immunologic, genetic and environmental factors are involved. It has been indicated that AA is an autoimmune T-cell-mediated disease with the involvement of some proinflammatory cytokines such as IL-1β, IL-15, IFN-γ, and TNF-α. Histologically, alopecia areata is characterized by the presence of lymphocytic infiltrate surrounding the bulb of the hair follicles [5, 42]. In the current study we investigated for the first time expression of S100A7 and S100A4 in AA lesional skin. However, we did not find any difference in the expression of these molecules in AA lesional skin as compared to normal skin of healthy controls. The expression of S100A7 was significantly lower in AA lesional skin as compared to LPP lesional skin. There are several studies addressing the role of IL-17 in the pathogenesis of alopecia areata. The majority of them focuses on the assessment of serum levels of interleukin-17 in patients with AA [43–46]. In a recent study, Tomaszewska et al. have indicated an elevated serum level of IL-17 in patients diagnosed with alopecia areata [43]. Nevertheless, in the literature there are also reports on the increased IL-17 expression in tissue samples obtained from patients with AA. Elela et al. showed that levels of IL-17 in skin biopsies were significantly higher in AA patients in comparison to controls [47]. Tanemura et al. found significantly increased immunoexpression of IL-17 in the dermis, particularly around hair follicles in lesional skin of patients with AA compared to healthy controls [48]. The results of our study are consistent with previous reports. We found significantly increased expression of IL-17 in AA lesional skin as compared to normal skin.
Conclusions
In the current study we showed, for the first time, the increased expression of S100A7 in LPP lesional skin, which may point towards an involvement of this molecule in the pathogenesis of LPP. Further, the results of our study highlight the possible role of IL-17 in the pathogenesis of LPP. Results of our study may help to develop new therapeutic methods for patients with LPP.