Introduction
Chronic lymphocytic leukemia (CLL) is a lympho-proliferative disease characterized by the accumulation of monoclonal CD5+ B cells in the bone marrow, blood and peripheral lymph organs. During the disease progression, a number of accompanying symptoms can occur including autoimmune phenomena, secondary malignancies, and increased susceptibility to infection. Infectious complications continue to be one of the leading causes of comorbidity and mortality in CLL patients [1, 2]. Accordingly, numerous defects in the patients’ immune system have been identified, including hypogammaglobulinemia, abnormalities of cellular immunity, defects of the complement system, and neutrophil phagocytic activity [3].
Beside CLL, various other lymphoproliferative disorders such as Hodgkin lymphoma and multiple myeloma are also commonly accompanied by immunodeficiency, and it has been recently suggested that galectin-1 (Gal-1) may be implicated in the development of such a condition [4-6]. Galectins are an evolutionarily conserved family of β-galactoside binding lectins, ubiquitously distributed in the organism, that can be found both intra- and extracellularly. In humans, 12 different galectin classes have been described, all of which have been proposed to play an important role in regulating the immune response [7]. Specifically, Gal-1 was shown to perform immunosuppressive functions by antagonizing activation signals derived from the T cell receptor (TCR) during T cell activation, but also by inducing apoptosis of activated T cells and expansion of regulatory T and B cells [8-12].
In CLL patients elevated plasma levels of Gal-1 were documented, and associated with disease progression, given that Gal-1 levels were higher in the Binet A clinical stage compared to the stage Binet C. Gal-1 levels were also higher in the patients with poorer disease prognosis based on the expression of ZAP70 and CD38 molecules [6]. Interestingly, activation of CLL clones in the presence of Gal-1 resulted in the production of the immunosuppressive cytokine IL-10, which is important in survival of the malignant cells [6]. Taken together, these results suggest that Gal-1 may be a relevant factor in the disease progression but also mediate the development of immunodeficiency.
Considering these data, we conducted an in vitro study in which we examined the effects of Gal-1 inhibition in CLL patients with clinically manifested immunodeficiency. The effects of Gal-1 inhibition were estimated in the experimental model of dendritic cells (DCs) co-cultured with allogeneic CD4+ T cells, considering that DCs are critical in mounting and shaping an adaptive immune response.
Material and methods
Dendritic cells cultivation and experimental protocols
The immunomodulatory properties of Gal-1 in the serum of CLL patients were tested in DC cultures obtained from peripheral blood mononuclear cells (PBMCs). Briefly, PBMCs were isolated from buffy coat of the healthy voluntary blood donor, using centrifugation over a Ficoll-Hypaque density gradient (Histopaque-1077; Sigma-Aldrich, St. Louis, Missouri, USA). Upon isolation, PBMCs were resuspended in 5 ml of cell culture medium – RPMI-1640 medium supplemented with 2.05 mM L-glutamine, 25 mM HEPES (Sigma-Aldrich, St. Louis, Missouri, USA), 10% inactivated fetal bovine serum (FBS) (Capricorn Scientific GmbH, Ebsdorfergrund, Germany) and 50 µM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, Missouri, USA), and then seeded into 25 ml cell culture flasks (30 × 106 cells per flask). After brief incubation at 37°C in 5% CO2 at 95% humidity for 90 min, supernatants were discarded and the flasks were rinsed repeatedly to eliminate non-adherent cells. Then, 5 ml of fresh RPMI-1640 medium with 10% FBS and 50 µM 2-mercaptoethanol, enriched with human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (100 ng/ml) and interleukin (IL)-4 (20 ng/ml) (both obtained from Sigma-Aldrich, St. Louis, Missouri, USA), was added in order to obtain DC cultures from monocytes adhered to the flask bottoms. On the third day, 2.5 ml of medium was replaced with the same amount of fresh, GM-CSF and IL-4 enriched medium. On the sixth day, DCs were seeded into 24-well plastic plates (8 × 105 cells per well in 2 ml RPMI-1640 media) and treated with the Gal-1 inhibitor OTX008 (other names: PTX008 or Calixarene 0118 (Axon Medchem, Reston. Virginia, USA)) alone at a concentration of 50 µM, 30% inactivated serum of the healthy donors and CLL patients with clinically manifested immunodeficiency, the combination of CLL serum and OTX008, and lipopolysaccharide (LPS) isolated from E. coli O111:B4 (Sigma-Aldrich, St. Louis, Missouri, USA). Using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, OTX008 concentration of 50 µM was determined not to be cytotoxic (data not shown). After cultivation for 48 h, the effects of sera on the alterations of DC phenotypic characteristics, their immunomodulatory properties and the ability to polarize the immune response were examined. The research was approved by the Ethical Committee of the Clinical Center Nis, Serbia under identification number 35110/11.
Sera used in the experiment
In the experiment, sera of healthy donors and CLL patients with clinically manifested immunodeficiency were used. Previously, Gal-1 serum level was determined using the Human Galectin-1 Quantikine ELISA kit (R&D Systems, Minneapolis, Minnesota, USA) according to the instructions provided. The demographic characteristics of the study participants are given in Table 1.
Table 1
Demographic characteristics of study participants
Evaluation of DC maturation markers
Dendritic cell phenotype alterations were investigated by the expression of DC maturation markers – CD80, CD83 and CD86 molecules, and the enzyme indolamine 2,3-dioxygenase 1 (IDO-1), using flow cytometry. After washing, the cells were incubated in stain buffer (BD Biosciences, Franklin Lakes, New Jersey, USA) for 30 min at room temperature with fluorochrome conjugated monoclonal antibodies. The following antibodies were used: anti-CD80FITC, anti-CD83FITC, and anti-CD86PE (R&D Systems, Minneapolis, Minnesota, USA). In order to block the non-specific antibody binding, stain buffer was supplemented with 10% human inactivated AB serum. For intracellular staining, cells were first permeabilized and fixed using a Fixation/Permeabilization Kit (BD Biosciences, Franklin Lakes, New Jersey, USA) according to the manufacturer’s instructions, and then stained with anti-IDO-1APC (R&D Systems, Minneapolis, Minnesota, USA). After washing, samples were analyzed on a BD LSRFortessa using BD FACSDiva software.
Evaluation of DC immunomodulatory properties
The effects of sera on the immunomodulatory properties of DC were evaluated by determining IL-2, IL-4, IL-10, IL-12 and transforming growth factor β (TGF-β) levels in cell culture supernatants. The following ELISA kits were used: Human IL-2 Quantikine ELISA Kit, Human IL-4 Quantikine ELISA Kit, Human IL-10 Quantikine ELISA Kit, Human IL-12p70 Quantikine ELISA Kit, and Human TGF-β Quantikine ELISA Kit (all obtained from R&D Systems, Minneapolis, Minnesota, USA) according to the manufacturer’s instructions.
Evaluation of DC ability to polarize the immune response
The study of serum effects on DC ability to polarize the immune response was conducted in the co-cultures with allogeneic CD4+ T cells. Human CD4+ T cells were isolated as a negative fraction by immunomagnetic sorting from PBMCs obtained from buffy coat of a healthy voluntary blood donor using an EasySep Human CD4+ T Cell Isolation Kit (Stemcell Technologies, Vancouver, Canada) according to the instructions provided. After isolation, 1 × 105 CD4+ T cells were co-cultured with 1 × 104 DCs in a 96-well U-bottom plate for 5 days. After the fifth day, cell cultures were treated with phorbol myristate acetate – PMA (20 ng/ml) and ionomycin (100 ng/ml) (Sigma-Aldrich, St. Louis, Missouri, USA) for 8 h to stimulate cytokine production. Supernatants were collected and IL-2, IL-4, IL-10, IL-17, interferon γ (IFN-γ) and TGF-β levels were determined using the following ELISA kits: Human IL-2 Quantikine ELISA Kit, Human IFN-γ Quantikine ELISA Kit, Human IL-4 Quantikine ELISA Kit, Human IL-10 Quantikine ELISA Kit, Human IL-17 Quantikine ELISA Kit, and Human TGF-β Quantikine ELISA Kit (all obtained from R&D Systems, Minneapolis, Minnesota, USA) according to the manufacturer’s instructions. In the remaining cells the frequency of T regulatory cells (Tregs) was estimated using flow cytometry. Cells were washed in stain buffer (BD Biosciences, Franklin Lakes, New Jersey, USA), and then permeabilized and fixed using Transcription Factor Phospho Buffer Set (BD Biosciences, Franklin Lakes, New Jersey, USA). To identify Tregs, anti-CD4FITC, anti-CD25APC, and anti-FoxP3PE antibodies (all obtained from BD Biosciences, Franklin Lakes, New Jersey, USA) were applied. After washing, sample analysis was performed on a BD LSRFortessa using BD FACSDiva software.
Statistical analyses
All statistical analyses were performed in SPSS v17.0 (SPSS Inc., Chicago, IL, USA). The results are presented as the mean values obtained from three experiments ± standard deviation (SD). The intergroup differences were established using one-way analysis of variance (ANOVA) with Tukey’s HSD test, and p values less than 0.05 were considered statistically significant.
Results
Effects of Gal-1 inhibitor (OTX008) on phenotype and immunomodulatory properties of DCs
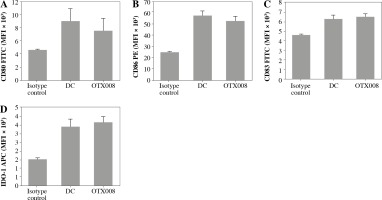
The allosteric inhibitor OTX008 was used to block Gal-1 in the CLL patient serum. By determining the expression level of DC maturation markers (CD80, CD83, CD86) and IDO-1, it was found that OTX008, per se, did not modify these phenotype characteristics of DCs (Fig. 1).
Fig. 1
Effects of OTX008 on DC maturation markers: DCs were treated with OTX008 (50 μM) for 48 h and then the expression level of DC maturation markers CD80, CD83, CD86, and IDO-1 was determined by flow cytometry (DC – untreated dendritic cells)
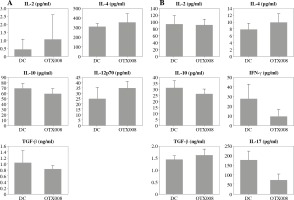
In DC co-cultures with allogeneic CD4+ T cells, OTX008 did not cause significant changes in the cytokine profile of DCs (Fig. 2A), or significant alterations in the CD4+ T cell polarization profile (Fig. 2B).
Fig. 2
Effects of OTX008 on DC cytokine profile (A) and their ability to polarize the immune response in co-cultures with CD4+ T cells (B): DCs were treated with OTX008 (50 μM) for 48 h then the cytokine levels were determined in the collected supernatants (A). Treated DCs were then co-cultured with CD4+ T cells for 5 more days, and prior cytokine determination, stimulated with PMA (20 ng/ml) and ionomycin (100 ng/ml) for 8h (B) (DC – untreated dendritic cells)
Effects of CLL serum on the phenotype and immunomodulatory properties of DCs
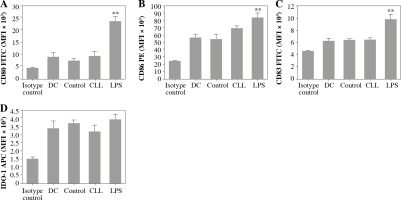
The effects of CLL serum on DC ability to modulate the immune response were estimated by their phenotype and cytokine profile, as well as the polarization profile of CD4+ T cells in co-cultures. It was found that CLL serum did not induce significant alterations in the expression of DC maturation markers, in contrast to LPS treatment (Fig. 3).
Fig. 3
Effects of CLL serum on DC maturation markers: DCs were treated with 30% inactivated serum of healthy subjects, CLL patients, and LPS (1 ng/ml) for 48 h. The expression level of DC maturation markers CD80, CD83, CD86, and IDO-1 was determined by flow cytometry (*p < 0.05, **p < 0.01 vs. CLL) (DC – untreated dendritic cells, Control – serum of healthy subjects, CLL – serum of CLL patients)
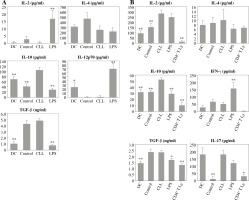
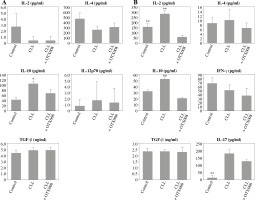
However, treatment with CLL serum resulted in a statistically significant increase in production of the immunosuppressive cytokine IL-10, in both pure DC cultures (Fig. 4A) and DC co-cultures with CD4+ T cells (Fig. 4B). At the same time, in the co-cultures, an increase in IL-2 production was observed (Fig. 4B). The production of other tested cytokines was not significantly different when compared to untreated DC and/or DC treated with control serum.
Fig. 4
Effects of sera on DC cytokine profile (A) and their ability to polarize the immune response in co-cultures with CD4+ T cells (B): DCs were treated with 30% inactivated serum of healthy subjects, CLL patients, and LPS (1 ng/ml) for 48 h, then cytokine levels were determined in the collected supernatants (A). Treated DCs were co-cultured with CD4+ T cells for 5 more days, and prior cytokine determination, stimulated with PMA (20 ng/ml) and ionomycin (100 ng/ml) for 8 h (B) (*p < 0.05, **p < 0.01 vs. CLL) (DC – untreated dendritic cells, Control – serum of healthy subjects, CLL – serum of CLL patients, CD4+ T Ly – CD4+ T cells alone)
Effects of Gal-1 inhibition on phenotype and immunomodulatory properties of DCs
Dendritic cells were also treated with the combination of CLL serum and OTX008 to evaluate the immunomodulatory effect of serum Gal-1, as well as the effects of its inhibition. No significant alterations of DC maturation markers were documented (Fig. 5).
Fig. 5
Effects of Gal-1 inhibition on DC maturation markers: DCs were treated with 30% inactivated serum of healthy subjects, CLL patients, and the combination of CLL serum and OTX008 (50 μM) for 48 h. The expression level of DC maturation markers CD80, CD83, CD86, and IDO-1 was determined by flow cytometry (Control – serum of healthy subjects, CLL – serum of CLL patients, CLL + OTX008 – serum of CLL patients in the combination with OTX008)

However, OTX008 treatment resulted in decreased production of IL-10 in both DC cultures (Fig. 6A) and co-cultures with CD4+ T cells (Fig. 6B) to levels that were comparable to the healthy control. In DC co-cultures with CD4+ T cells reduced production of IL-2 was also observed following OTX008 treatment. Interestingly, the treatment with control serum significantly decreased the IL-17 level compared to the treatment with CLL serum with or without OTX008 (Fig. 6B).
Fig. 6
Effects of Gal-1 inhibition on DC cytokine profile (A) and their ability to polarize the immune response in co-culture with CD4+ T cells (B): DCs were treated with 30% inactivated serum of healthy subjects, CLL patients, and the combination of CLL serum and OTX008 (50 μM) for 48 h, then cytokine levels were determined in the collected supernatants (A). Treated DCs were co-cultured with CD4+ T cells for 5 more days, and prior cytokine determination, stimulated with PMA (20 ng/ml) and ionomycin (100 ng/ml) for 8 h (B) (*p < 0.05, **p < 0.01 vs. CLL + OTX008) (Control – serum of healthy subjects, CLL – serum of CLL patients, CLL + OTX008 – serum of CLL patients in the combination with OTX008)
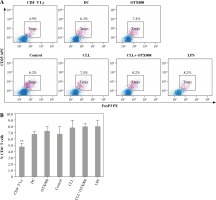
Effects of Gal-1 inhibition on Treg expansion
In order to examine the potential immunosuppressive properties of serum Gal-1, Treg frequency was determined in DC co-cultures with CD4+ T cells, but no statistically significant intergroup differences were observed (Fig. 7).
Fig. 7
Effects of Gal-1 inhibition on Treg expansion, representative results of one experiment (A); percentage of Treg in co-cultures (B): DCs were treated with OTX008 (50 μM), 30% inactivated serum of healthy subjects, CLL patients, the combination of CLL serum and OTX008 (50 μM), and LPS (1 ng/ml) for 48 h. Treated DCs were co-cultured with CD4+ T cells for 5 more days and then Treg frequency was assessed by flow cytometry, using identification markers CD4, CD25, and FoxP3 (**p < 0.01 vs. CLL) (CD4+ T Ly – CD4+ T cells alone, DC – untreated DC, Control – serum of healthy subjects, CLL – serum of CLL patients, CLL + OTX008 – serum of CLL patients in the combination with OTX008)
Discussion
Considering the well-known immunosuppressive properties of Gal-1 on the constituents of both innate and acquired immunity [13], in the conducted study, we examined the relevance of Gal-1 in the pathogenesis of immunodeficiency in the patients with CLL, as previously suggested in other lymphoproliferative diseases [4, 14]. DCs play a key role in defining the characteristics of the acquired immune response, so we treated DCs with sera of healthy subjects and CLL patients with clinically manifested immunodeficiency in order to evaluate the effects of serum Gal-1 as well as the effects of its blockade with the allosteric inhibitor OTX008.
OTX008, per se, did not exert significant immunomodulatory properties, given that it did not alter the phenotype characteristics of DCs, their cytokine profile or ability to polarize the immune response when co-cultured with allogeneic CD4+ T cells.
By examining the effects of patients’ sera on the immunomodulatory properties of DCs, no significant changes in the expression level of DC maturation markers were documented. The expression of IDO-1, which is suggested to be an important indicator of the tolerogenic DC phenotype [15], was also unaltered. On the other hand, a statistically significant increase in the production of the immunosuppressive cytokine IL-10 was present in both pure DC cultures and co-cultures with CD4+ T cells. OTX008 was able to decrease IL-10 production to a level that was no longer different from that in healthy subjects. Such a finding might indicate that serum Gal-1 primes DCs to polarize the immune response toward enhanced IL-10 production. Beside CLL, similar effects of Gal-1 on DCs have been reported in the literature. In particular, Ilarregui et al. observed that CD4+ T cells when co-cultured with DCs pretreated with LPS and Gal-1 produced a significantly higher amount of IL-10 than those co-cultured with DCs pretreated with LPS alone [16]. In order to identify the potential phenotype of IL-10 producing CD4+ T cells induced by the serum of CLL patients, we examined the frequency of Tregs, identified as CD4+ CD25+ FoxP3+ T cells, but no significant intergroup variations were observed. This indicates that Gal-1 from sera of CLL patients could modulate DC phenotype in a manner that promotes CD4+ T cell differentiation toward IL-10+ but FoxP3– phenotype. Potential candidates could be type 1 regulatory T cells (Tr1), which exert immunosuppressive functions via IL-10 production but do not express FoxP3 as a basic transcription factor [17]. Interestingly, other authors have also shown that pretreatment of DC with Gal-1 did not lead to the expansion of conventional Treg yet stimulated differentiation of IL-10+ CD4+ T cells that had the ability to suppress T cell proliferation in a dose-dependent manner, whereby this effect was lost by the use of IL-10 neutralizing antibodies [16]. By direct binding to the CD45 molecule on the T cell surface, Gal-1 could also enhance IL-10 secretion and, at the same time, increase the expression of inhibitory co-stimulators including cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death 1 (PD-1) [18]. In CLL, elevated serum levels of IL-10 were indeed observed and correlated with biochemical parameters of the disease progression [lactate dehydrogenase (LDH), β2 microglobulin], disease clinical stage and overall survival [19]. In addition, IL-10 is a cytokine directly involved in the disease progression considering that it has been shown that IL-10 could impair the antitumor activity of the immune system against the malignant CLL clone [20]. Unfortunately, currently there are no literature data regarding the number and function of Tr1 in CLL patients.
Another significant finding of our study was that DCs pretreated with the sera of CLL patients enhanced IL-2 production when co-cultured with CD4+ T cells. This effect was neutralized by OTX008, suggesting Gal-1 involvement in this process. Such a finding may seem paradoxical considering that IL-2 is the main factor in promoting the T cell mediated immune response. However, regardless of its immunosuppressive effects, Gal-1 was also shown to stimulate the proliferation of naive T cells, but the significance of IL-2 in this process was not examined, and nor was the phenotype of expanded cells [21]. Accordingly, IL-2 should be reviewed in the broader context of the microenvironment and the presence of other signals, considering that it might exert dual functions – in addition to the expansion of effector T cells, IL-2 could also stimulate the expansion of T cells with immunosuppressive functions [22, 23]. In particular, IL-2 induced expression of transcription factor STAT-5 has been shown to stimulate IL-10 production in FoxP3– T cells [24], and in vitro induce Tr1 expansion [25]. However, the production of IL-2 in Tr1 is still controversial. Some authors claim that these cells produce relatively small amounts of this cytokine [26], but there are also data suggesting that IL-2 secretion by Tr1 allows the expansion of conventional Treg, and thus it is important for immunosuppressive properties of Tr1 [27].
The inhibition of serum Gal-1 in patients with CLL in DC co-cultures with CD4+ T cells did not significantly alter the secretion of IL-4, IL-17, TGF-β, and IFN-γ. In concordance, others have also indicated that DC pretreatment with Gal-1 did not modify IL-4 and TGF-β levels when co-cultured with CD4+ T cells. However, in contrast to our study, it was suggested that Gal-1 exerts immunosuppressive effects at least partially by suppressing the production of IFN-γ [16]. Another interesting finding was enhanced IL-17 production in co-cultures with CLL serum with or without OTX008. Although suggesting that Gal-1 has no influence on IL-17 production, this finding may indicate that IL-17 is an important factor in CLL pathogenesis, and some supporting data were reported [28, 29].
Conclusions
The results of our study suggest that Gal-1 could be implicated in the development of immunodeficiency in CLL patients by promoting alterations in DC phenotype that support the differentiation of a specific subset of IL-10 secreting CD4+ T cells, an effect that could be blocked by the Gal-1 inhibitor OTX008.


