Introduction
Alopecia areata is a condition that affects hair follicles and leads to hair loss ranging from small well-defined patches to complete loss of all body hair. Despite its high incidence, the pathobiology of this chronic hair-loss disorder is not fully understood, and the available therapies are symptomatic and not preventing a relapse of the disease [1, 2].
This review focuses on immunology of alopecia areata and defines key characteristics of the pathologic abnormalities of hair follicles. To date, available evidences has been suggesting that alopecia areata is an autoimmune disease, in which the collapse of hair follicles immune privilege may play a crucial role in the pathogenesis [3, 4].
Normal hair growth
Hair follicles undergo lifelong, cyclic transformation [5]. Traditionally, three phases of hair growth were recognized, including anagen, with classification ranging from stages I to VI (a period of very rapid growth, pigmentation, and hair-shaft production), catagen (regression phase), and telogen (resting phase) [5]. This cyclic transformation is controlled by finely tuned changes in the local signaling milieu, based on changes in the expression of cytokines, hormones, neurotransmitters as well as transcription factors and enzymes. The regenerative capacity of hair follicles depends on a group of keratinocyte and melanocyte steam cells that are located in the follicular bulge area [5-7]. It is a convex protrusion of outer root sheath in the most distal permanent portion of hair follicle, just below the sebaceous gland. Regulation of bulge cell activities involves many signaling pathways, such as Wnt/β-catenin, BMP/Smad, and Sonic hedgehog (Shh) signaling [8-10].
Immunobiology of the hair follicle
Decades ago, it was reported that hair follicles establish a special area, which enables transplanted allogeneic cells to escape detection and elimination by the immune system [11]. A creation of relative immune privilege protects from an autoimmune attack on intrafollicularly expressed autoantigens and prevents an immunopathogenic injury [12]. Several mechanisms collaborate in providing immune protection (Table 1). A unique feature of hair follicle immune privilege is that it demonstrates the unusual ability to completely regenerate itself during each anagen phase. Additionally, it is limited only to those parts of the hair follicle epithelium, which are generated from hair follicle steam cells (i.e., hair matrix and inner root sheath) [5, 13].
Table 1
The mechanisms of HF immune privilege
| Low number of CD4+ or CD8+ T cells and CD1a+ Langerhans cell in anagen hair bulb. Additionally, Langerhans cells have a reduced MHC class II-dependent antigen presenting capacity bulb [98] |
|---|
| Expression of MHC I in anagen hair bulbs is absent or substantially reduced [99] |
| Melanocytes of the hair follicle pigmentary unit are MHC I negative [98] |
| Anagen hair follicles contain very few NK cells and they do not express MICA [46] |
| Interferon regulatory factor-1 reactivity is significantly down-regulated in the anagen hair matrix (interferon-γ acts as the most important enhancer of MHC I expression via interferon regulatory factor-1) [47] |
| Anagen hair bulb generates immunosuppressive molecules, such as TGF-β1, TGF-β2, ACTH, and α-MSH [100, 101] |
| Epithelial hair bulb is unsheathed by a special matrix barrier, which hinder immune cell trafficking [102] |
Epidemiology
The lifetime risk of alopecia areata is about 1.7% in the general population. The prevalence of alopecia areata ranges from 0.1% to 0.2% worldwide, depending on the ethnic background [14]. It affects approximately 0.7% to 3.8% of all the patients attending dermatology clinics [15]. The peak incidence of alopecia areata seems to occur among 15 to 29-year-old individuals. Onset of alopecia areata occurs in 44% of patients before their 20s, and less than 30% develop the disease after their 40s [15, 16]. The clinical course is highly unpredictable, and it can occur at any age from birth to the late decades of life.
Clinical features
Most commonly, alopecia areata manifests as a rapid hair loss in small, well-circumscribed patches. The lesions are usually round or oval, with distinctive borders, and normal hair delineating the periphery. The hair loss appears in isolated or several patches, and scalp is the most common site affected by alopecia areata (90%) [17, 18]. The disease may lead to complete baldness (areata totalis) or loss of all body hair (alopecia universalis). Several variants of alopecia areata have been described. Ophiasis clinically manifests as hair loss in the shape of a wave at the circumference of the head. ‘Sudden graying’ is a variant, in which very sudden, overnight graying is caused by preferential loss of pigmented hair. A very rare type is alopecia areata diffusa, characterized by diffuse (non-patchy) hair loss with female predominance [19]. Alopecia areata incognita, with loss of hair primarily in the vertex area, is considered by some authors a subtype of alopecia areata [19]. Spontaneous hair regrowth has been described in individual cases; however, in most cases, long-term immunosuppressive or immunomodulating therapy is required. In alopecia areata, the regrowing hairs are often white, becoming pigmented during growing. In severe forms, hair loss may persist for many years.
Characteristic trichoscopy features that indicate areas of activity are ’exclamation-mark’ hairs (when the distal segment of hair shaft is broader than its proximal end) and ‘black dots’ (hairs that have broken at the scalp surface) [20, 21]. Because alopecia areata is a systemic disorder, it can also affect nails and eyes. Nail changes (beau lines, nail pitting, onychomadesis, onycholysis, trachyonychia, and hemorrhagic spotting of lunula) may be seen in 3-30% of patients [22]. Their involvement is usually associated with severe alopecia areata.
Pathobiology and immunology
The pathobiology of alopecia areata remains unclear. Currently, little is known about risk factors, but various studies have suggested that alopecia areata is a complex multigenetic trait with an inherited predisposition component [23]. Moreover, discordance in identical twins indicated that the disease could be triggered by environmental factors [24]. The majority of cases are sporadic, and the reported frequency of a positive family history varies from 3% to 42% [25, 26].
Alopecia areata is mostly considered to be a cell-mediated autoimmune disease, in which autoreactive cytotoxic T cells recognize melanocyte-associated proteins, such as tyrosinase. On the other hand, some evidences suggest that it is not a truly autoimmune disease but is only ‘consistent with’ autoimmune mechanism [27]. Below we analyze these two hypotheses.
Alopecia areata as an autoimmune disease
Direct and indirect evidences support hypothesis that alopecia areata is a tissue-specific autoimmune disease with a genetic predisposition and an environmental trigger. Recent study shows a higher incidence rate in the female population [28]. The disease is associated with increased risk of other autoimmune disorders (Table 2). Approximately, 12-16% of individuals with alopecia areata develop an autoimmune disease [29, 30].
Table 2
Autoimmune diseases associated with alopecia areata
| Autoimmune disease | References |
|---|---|
| Hashimoto thyroiditis | [103] |
| Vitiligo | [104-106] |
| Systemic lupus erythematosus | [26] |
| Autoimmune thrombocytopenic purpura | [107] |
| Type I (insulin-dependent) diabetes | [79] |
| Myasthenia gravis | [105] |
| Celiac disease | [108] |
| Scleroderma | [106] |
| Ulcerative colitis | [105, 106] |
Histologic feature of alopecia areata is a lymphocytic infiltrate adjoining the hair follicle site, which may appear in a characteristic “swarm of bees” pattern [31]. This is a result of the collapse of hair follicle immune privilege, with exposure of autoantigens that lead to an accumulation of autoreactive T cells [32]. An infiltration is observed, particularly in the acute stage of the disease. In the accumulation surrounding the hair follicle, CD4+ Th1 cells predominate, while within the follicular epithelium, CD8+ Tc1 cells represent the majority [33]. In the chronic stage, marked hair follicle miniaturization and cell accumulation decrease are observed, but CD8+ Tc1 cells are still present [34].
Round alopecia lesions may reflect a chain autoimmune reaction in adjacent hair follicles induced by T cell and cytokine diffusion [27]. CD8+ T cells can be differentiated into two phenotypes (Fig. 1). Type I - CD8+ cytotoxic T (Tc1) cells secrets IFN-γ and play an essential role in the development of autoimmune diseases, such as autoimmune thyroiditis [35]. A significant number of those cells in skin lesions of alopecia areata contribute to cell-mediated autoimmune reactions. In addition, the proportions of CD4+ Th1 cells and CD8+ Tc1 cells are considerably higher in the peripheral blood mononuclear cells (PBMCs) [36]. These cells display strong chemotactic activity towards CXCL10 [34]. Also, the Th1 chemokines (CXCL9, CXCL10, and CXCL11) are highly expressed in alopecia areata lesions [37]. It has been suggested that improved production of CXCL10 from hair follicles induces preferential infiltration of Th1 and Tc1 cells in the acute phase of alopecia areata, and Tc1 infiltration remains prolonged within the chronic phase [34]. In addition, an overall impairment in the Th1-mediated immune response suggests increased IL-2 and IFN-γ serum levels [38]. IL-2 shows a positive correlation with duration and severity of the disease [38]. Observed efficacy of JAK inhibitors including ruxolitinib, tofacitinib, and baricitinib in the treatment of alopecia areata appears to be secondary to the disruption of Th1 immune response and elimination of IFN-γ effect [39, 40]. Preclinical studies have also shown that abatacept, a recombinant fusion protein (CTLA4-Ig) is effective in preventing the disease in mouse model [41]. Abatacept blocks the potential co-stimulatory interaction with antigen-presenting cells/APC, a process required for full T cell activation.
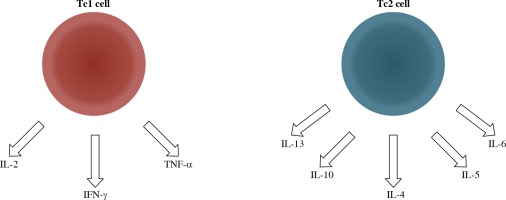
Fig. 1
Effector CD8+T cells can be segregated into two populations on the basis of the cytokines they secrete similar to CD4+ T cells. Tc1 cells characteristically produce type-1 cytokines (IL-2, TNF-α, and IFN-γ), whereas Tc2 cells secrete type-2 cytokines (IL-4, IL-5, IL-6, IL-10, and IL-13). Tc1 cells as well as Th1 cells are involved in the protection against intracellular parasites and delayed-type hypersensitivity, but also, they play an important role in autoimmunity. Autoimmune diseases are characterized by increased Tc1/Tc2 ratio, in the same way as Th1/Th2 ratio
Hair follicle is a site of immune privilege [11]. Immunoprotection is limited to the anagen, sequesters melanogenesis, and other anagen-associated antigens of immune recognition [42]. In catagen, the hair follicle regresses, and involves apoptosis and remodeling of the transient portion [43]. Immune cells infiltrate around catagen-stage hair follicles [44]. This process cyclically exposes the immune system to low levels of hair follicle’s antigens. If catagen regression became disordered, inappropriate expression of stimulatory antigen to the immune system might breach the threshold for the onset of overt alopecia areata [45]. Many hypotheses for autoimmunity onset in alopecia areata have been reported [27]. The most widely accepted is the immune privilege collapse theory [42]. It postulates that the disease occurs only when key events coincide (Fig. 2) [12].
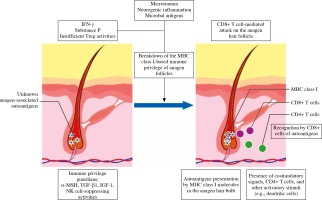
Fig. 2
Proposed pathogenesis of alopecia areata. The immunosuppressive milieu of the anagen hair bulb is modulated by immunosuppressive factors, such as α-MSH, TGF-β, and IGF-1, and suppressing NK-cells. Alopecia areata is hair follicle response pattern to various inflammatory events associated with e.g., IFN-γ-induced up-regulation of expression of MHC class Ia inside the hair follicle. Anagen-associated autoantigens are recognized, once they become exposed by ectopic MHC class I. That leads to autoimmune reactions against hair follicle
MHC class I-negative hair follicles are protected from NK cell attack by MICA-negative outer root sheath, low expression of NKG2D on NK cells, and inhibitory KIRs. In alopecia areata, NKG2D+ NK cells attack MICApositive hair follicles, which results in apoptosis and hair loss [46]. Also, the number of NK cells that express NK cell-inhibitory KIR2DL2 and KIR2DL3 is significantly decreased in patients [46].
Locally generated immunosuppressants (e.g., α-MSH, TGF-β1, and IGF-1) [47] and NK cellsuppressing activities (e.g. MIF expression) [48] provide hair follicle immune privilege. Insufficient activity of these agents predisposes towards development of alopecia areata [12]. Inflammatory cytokines, including substance P and IFN-γ, lead to the collapse of immune privilege by upregulation of expression of MHC class Ia inside the hair follicle. IFN-γ is associated with a Th1 cellular infiltrate and Tc1 cells are the main source. In addition, alopecia areata can sometimes be triggered or exacerbated by viral infections that cause excess secretion of interferons (IFN) [49, 50]. The background of autoimmunity is molecular mimicry and epitope spreading [51]. Also, systemic treatment with interferon-alfa has been linked to the exacerbation or onset of patchy alopecia areata [52, 53], and type 1 interferon--related proteins are overexpressed in the inflammatory lesions [54]. Moreover, TNF-α level is significantly elevated both in lesional and non-lesional skin biopsies of patients [55].
Another concept is the role of IL-17-producing cells. T helper 17 cells have been characterized as a novel subset of CD4+ T cells that produce IL-17A, IL-17F, and IL-22. They are involved in the pathogenesis of autoimmune diseases [56]. Elevated IL-17 levels were found in rheumatoid arthritis, systemic lupus erythematosus, and psoriasis [57]. Recent reports suggest the contribution of IL-17 to the development of alopecia areata. IL17RA gene polymorphism is associated with increased susceptibility to alopecia areata [58]. Additionally, Th17 cells are present in affected lesions, particularly around hair follicles [59]. A study suggests that the contribution of IL-17-producing cells is even stronger than that of IFN-γ-producing cells in the pathogenesis [60]. These cells might enhance the local inflammation in combination with decreasing T reg cells in the affected skin of alopecia areata. Interestingly, the ratio of IL-17-producing cells in acute, diffuse, and total alopecia is significantly lower than in multiple patchy types of alopecia areata [60]. Therefore, the ratio of IL-17-producing cells in the lesional skin might be connected with clinical types of the disease. Denser Th17 cells infiltration is associated with more severe symptoms in comparison with a longer duration of the disease [61], which may be explained by long survival of Th17 cells in chronic inflammation. Cytokine that plays a role in the in the maintenance of Th17 response is IL-23. At the moment, three agents that block IL-17 are investigated, including secukinumab, brodalumab, and ixekizumab [62]. However, most of mentioned evidences to support the role of IL-17-producing cells in alopecia areata are not strong (see below).
Antibodies can be found in abundance in alopecia areata, sometimes in extremely high titers. These alopecia areata antibodies are useful in search for target antigens. They react with multiple components of anagen hair follicles [63, 64]. It is known that alopecia areata almost solely attacks growing (anagen) hair follicles that engage in active melanogenesis [65]. Pigmented hair follicles are selectively lost in active disease, whereas regrowing hair are frequently white. Alopecia areata is also often associated with vitiligo [63, 66]. These observations suggest involvement of melanogenesis-associated autoantigens. In addition, follicular melanocytes show histological abnormalities and in some affected hair bulb are completely deleted [11]. Finally, in a late stage of alopecia areata, epitope spreading occurs, developing from autoantibodies to innumerable epitopes [67]. Another suggested mechanism is an immune response against a variety of hair keratins [68].
Autoimmunity in alopecia areata is strongly supported by effectiveness of immunosuppressive agents. Good response is achieved with allergic contact sensitizers (e.g., diphenylcyclopropenone), which are applied to achieve low-grade chronic dermatitis [69]. Their mechanism of action probably include diversion of the T cell response from the hair follicle to epidermis, and induction of localized immunosuppression by production of immunosuppressive cytokines (e.g., TGF-β, IL-10, and myeloid-derived suppressor cell) [70, 71]. Benefit for the treatment may provide also other immunosuppressive agents, especially systemic corticosteroids and cyclosporine [72]. The newest idea for the alopecia areata therapy is application of JAK inhibitors, such as ruxolitinib and tofacitinib [73, 74]. Systemic and topical administration was highly effective in reversing disease and associated with a markedly reduced proportion of CD8+ NKG2D+ cells.
Alopecia areata as not a truly autoimmune disease
The characteristics of alopecia areata vary from those seen in classical autoimmune diseases. In contrast to other autoimmune diseases, when women are affected three times more than men, a study report equal frequency in both sexes [75]. The peak prevalence occurs at the age of 20, but it can affect patients at any age [76]. The fact that the concordance rate in monozygotic twins is not 100% indicate a complex nature of the disease, involving both genetic and environmental triggers [77]. In addition, literature indicates that coincidence of other autoimmune diseases does not differ significantly [78]. For example, frequency of type 1 diabetes mellitus is reduced in patients, but significantly increased in their relatives [79].
The mononuclear cell infiltrate around the terminal hair bulb is believed to be the classic finding in the early (acute) stage of alopecia areata [80]. However, this infiltrate is found in only one-third of patients, which are initially present with alopecic patches. Furthermore, immune cells are commonly absent in biopsies taken at a later stage [80] and in areas of severe follicular damage [63]. Accepting the disease targets is a hair follicle-associated antigen, and patients should have an abundance of lesions, such as in alopecia totalis or universalis, not single, circumscribed patches. Additionally, there are long periods of complete clinical remission that are not pathognomonic for autoimmune diseases [27].
Administration of IFN-γ has previously been suggested to promote a collapse of hair follicle immune privilege that may initiate inflammatory lesions in alopecia areata [81]. Unfortunately, some laboratories report that the occurrence of alopecia areata after IFN-γ injections did not changed the frequency [82]. It shows that although hair follicle immune privilege collapse may be required to initiate disorder, additional factors are also needed. Several immune privilege genes are downregulated in perilesional and lesional hair follicles [83]. They suggest that aberration in hair follicle immune privilege is not sufficient to initiate the disease.
A concept that melanogenesis-related proteins are the source of initiating epitopes is the most widely accepted. Unfortunately, observations supporting this thesis are based on data obtained from patients with clinically established disorder, not at the time of initiation. The antigen targeted at the time of alopecia areata onset may not be the same as the epitope to perpetuate hair loss in chronic disease. Moreover, alopecia areata may occur in albino strains of mice [84]. Although they are unable to produce ultimate melanin pigments, the biochemical pathway to produce melanin, including melanosomes, is normal. This data suggest that melanin-related proteins are not the inciting epitope, but they may occur later. Additional unresolved question is the role of hair follicle autoantibodies. Failure to inhibit hair growth in human scalp skin, grafted onto nude mice by passive transfer of autoantibodies from affected individual suggest that they are not always the key pathogenic factor [85]. Autoantibodies against a variety of hair keratins can be also found, to various degrees, in clinically normal patients [68, 86]. There is no consistent correlation between the development of these autoantibodies and the onset of alopecia areata.
Environmental insults, such as infection, contribute to autoimmunity and they have been discussed as possible trigger factors. However, the association of cytomegalovirus (CMV) has been refused [24]. The detection of CMV DNA sequences in skin biopsies seems to be an incidental finding. Similarly, other viruses, including hepatitis B (HBV), hepatitis C virus (HCV), or even vaccinations have been suggested to trigger alopecia areata [87, 88]. A study with C3H/HeJ mouse model for adult onset alopecia areata indicated no effect of the vaccine [89].
The beneficial response to immunosuppression therapies implies an autoimmune mechanism. However, many inflammatory diseases also benefit from immunosuppression, so this fact should not be considered alone as evidence of an autoimmune etiology. Although, new biological drugs are effective therapies for numerous autoimmune diseases in alopecia areata, no significant efficacy was shown and some studies report on aggravation during therapy [90, 91]. This paradoxical side effect could occur because of the fact that down-regulation of TNF-α may up-regulate IFN-γ [92].
Conclusions
Alopecia areata is a recurrent form of non-scarring hair loss. Over the last two centuries, many hypotheses have been proposed to explain pathogenesis, none of which have been proven so far. Most authors tend to classify alopecia areata as an autoimmune disease [93]. However, this concept in only supported by circumstantial evidence, and no theory is accepted in general. This is especially true when considering Witebsky’s postulates [94]. Autoantibodies to hair follicle-associated antigens occur in normal individuals, and no specific follicular epitope has been identified as responsible for triggering alopecia areata. Until the antigen is found, the autoimmune concept still remains a hypothesis. Several other theories are occasionally revisited in the medical literature. For example, recent studies emphasized the importance of genetic factors [23, 27, 95]. Psoriasis, which is also suggested to be an autoimmune-mediated disease, has a strong genetic basis. The genome-wide association studies have clearly implicated the role of both innate and adaptive immune systems in the pathogenesis of the disease. There are also multiple reports of alopecia areata development in recipients of allogeneic bone marrow transplantation from affected HLA-matched donors [96, 97]. All these data suggest that alopecia areata has a strong immune component, although other factors may play a role in a disease etiology. Furthermore, in different subsets of alopecia areata, autoimmune, genetic, and infectious factors come together with a different weight in triggering the pathology. Further studies should be performed in patient groups with documented subtypes of alopecia areata to elucidate the underlying mechanisms of pathways involved in pathogenesis of alopecia areata.


