Introduction
Urticaria constitutes a heterogeneous group of diseases characterized by the occurrence of urticarial wheals, which may be associated with angioedema symptoms. According to its definition, chronic urticaria involves wheals recurring over 6 weeks [1]. Chronic spontaneous urticaria (CSU) is a disease with complex etiology, which cannot be determined in many cases. In recent years, numerous studies have been conducted in order to evaluate pathomechanisms causing the disease. Among potential factors that may play a role in CSU pathogenesis, one may mention autoimmune processes, chronic infections, pseudoallergies, and presence of a chronic inflammatory condition, which is observed, among others, in obesity [2-4].
Fat tissue constitutes a source of biologically active substances – adipokines, which – thanks to their proinflammatory action – may activate mast cells, leading to wheal development. One of the main hormones in the fat tissue is adiponectin, a protein with proven anti-atherosclerotic, anti-diabetic, and anti-inflammatory effects in the course of metabolic and cardiovascular diseases [5]. Studies of recent years suggest an unfavorable, pro-inflammatory role of this hormone in the development of autoimmunological diseases [6]. The effect of adipokines on the development and course of CSU has not been determined so far. In this work, the authors made an effort to assess the potential relationship between adiponectin concentration and CSU.
Material and methods
The study included 52 CSU patients (34 women and 18 men; average age, 40.63 ±12.39) (TotU). The patients were divided into two subgroups: 25 patients with wheals as the only visible symptoms (U), and 27 patients with angioedema symptoms apart from wheals (U-Ae). The disease was diagnosed on the basis of whole clinical picture, excluding coexisting diseases. The urticaria activity score (UAS) seven-day monitoring was used to assess disease intensity. This questionnaire calculates the number of wheals and the intensity of pruritus. The control group was composed of 43 healthy volunteers (29 women and 14 men; average age, 39.21 ±11.57). The adiponectin concentration was determined in blood serum (R&D), and body mass index (BMI) was calculated for the whole group and each subgroup.
Mann-Whitney U-test was used in statistical calculations. The study was approved by the Bioethics Committee of the Medical University of Silesia in Katowice, Poland. All subjects provided written consent.
Results
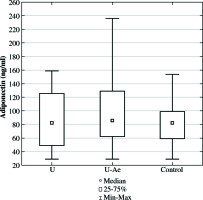
The comparison of the whole tested group with the control group did not show any statistically important difference in adiponectin concentration (Table 1). Similarly, no statistically important differences were found in adiponectin concentration, when comparing the two subgroups of the study group and controls (Table 1, Fig. 1). Additionally, no differences were observed in BMI and UAS values (Table 1).
Table 1
Adiponectin concentration, body mass index (BMI), and urticaria activity score (UAS) values in studied groups and subgrou ps
Discussion
Among potential factors that participate in the CSU pathogenesis, one may mention the presence of a chronic inflammatory condition observed, among others, in obese patients, particularly in a group with concurrent metabolic syndrome [7]. Epidemiologic studies of Italian population showed obesity as more frequently occurring in CSU patients, whereas results of Polish studies show a relationship between overweight and obesity and CSU occurrence and durability of CSU symptoms [8, 9]. Moreover, the frequency of metabolic syndrome occurrence is significantly higher in CSU patients comparing with healthy people [10]. The inflammatory process in the course of obesity manifests itself with an increased level of concentration of pro-inflammatory cytokines, including TNF-α, IL-6, and CRP [5].
Fat tissue constitutes a source of biologically active substances called “adipokines”, which play multidirectional functions in human body and include cytokines, growth factors, and hormones. In literature regarding this subject, many works emphasizing the relationship between adipokines and the development of type 2 diabetes, atherosclerosis, and metabolic syndrome can be found. However, the role of adipokines in CSU pathogenesis has not been fully explained. Determination of the relationship between adipokines and CSU seems to be of particular importance in the view of advancing obesity epidemics in highly developed countries.
Adiponectin is a protein generated mainly by white fat tissue, which occurs in 3 isoforms in the serum (LMW – low molecular weight, MMW – middle molecular weight, HMW – high molecular weight). The HMW fraction is mainly responsible for the biological activity of adiponectin [11].Studies show that adiponectin concentration is negatively correlated with BMI, insulin resistance, and fat tissue content [12]. Protective role of adiponectin in the course of metabolic and cardiovascular diseases was confirmed in numerous reports. Adiponectin plays a significant role in glucose metabolism, for instance, through decreasing gluconeogenesis and increasing insulin sensitivity of peripheral tissues. Moreover, it has a vasoprotective effect, stimulating generation of nitric oxide, inhibiting the effect of proinflammatory cytokines and adhesive molecules as well as reducing proliferation of endothelial cells and smooth muscle cells. Additionally, adiponectin presents an antiatherogenic effect, protecting all stages of atherosclerosis development.
The anti-inflammatory effect of adiponectin in the course of metabolic diseases is characterized by complex and multidirectional nature. Clinical studies have shown the suppressive effect of adiponectin on IL-6 and TNF-α secretion, through macrophages, upon prior stimulation with the lipopolysaccharide vaccine (LPS) as well as the effect of IL-10 increasing secretion and antagonists of IL-1 receptors through dendritic cells and monocytes [13, 14]. On the other hand, studies conducted on the group of obese and diabetic patients demonstrated that TNF-α and IL-6 have a suppressive effect on the production and multimerization of adiponectin, which indicates the presence of negative feedback between adiponectin and proinflammatory cytokines [15-18]. CSU is characterized by the presence of a chronic inflammatory condition, expressed by increased concentration of circulating proinflammatory factors such as IL-6, TNF-α, IL-1β, and CRP [4]. Irrespective of released preformed mediators, the activated mast cells produce a series of cytokines including Il-6, TNF-α, VEGF, or PAF, which may affect the level of adiponectin concentration in the blood [19]. The fact that the level of adiponectin in our study did not diverge from the one observed in the control group suggests the complexity of mechanisms regulating adiponectin concentration, including those independent from the course of CSU.
Additionally, a potential role in the CSU pathogenesis is assigned to an excessive activation of coagulation system. In animal models, it was observed that thrombin may induce degranulation of mast cells and increases permeability of blood vessels. Moreover, high concentration of D-dimers and FDP correlates with disease severity [20-22]. Adiponectin is characterized by anticoagulant effect; its concentration is negatively correlated with the fibrinogen and PAI-1 levels [23].
In CSU pathogenesis, some authors indicated an important role of oxidative stress. Rahu et al. observed increased activity of superoxide dismutase, glutathione, and malondialdehyde within urticaria eruptions in CSU patients comparing with control group, with no difference in their activity within the area of skin without lesions [24]. In a study on a pediatric population, Dilek detected increased level of total oxidant status of plasma in CSU patients, when compared with control group [25].However, other authors did not confirm this correlation [26]. One of the characteristics of adiponectin is its anti-oxidant effect. The studies showed that adiponectin concentration in blood is negatively correlated with the level of oxidative stress markers [27, 28].
Studies of recent years, indicating the proinflammatory effect of adiponectin in the course of autoimmunological diseases stand in opposition to the protective role of adiponectin in the development of obesity-related diseases. Increased adiponectin concentration was observed in patients with type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, or inflammatory bowel diseases [29, 30]. Hence, studies on the role of adiponectin in CSU pathogenesis seem to be of particular importance. Immunological system dysfunctions, including autoimmunological processes, constitute the most commonly occurring reason of incorrect activation and degranulation of mast cells in CSU. Comparability of adiponectin concentration in blood observed in the groups under study may result from heterogeneous character of the factors underlying the disease. According to literature, antibodies directed against α subunit of receptor with high connection with the Fc fragment of IgE antibody (anti-FcεRIα) and anti-IgE antibodies are present only in 30-50% patients with CSU. In other patients, the share of other mechanisms responsible for mast cell degranulation is considered, including the effect of pseudoallergens or chronic inflammatory process.
The anti-inflammatory and proinflammatory effect of adiponectin depends on the proportion of its isoforms in the blood. HMW adiponectin has mainly proinflammatory effect through inducing the production of IL-6 by monocytes [31]. In our study, we did not analyze the differences in the concentrations of various adiponectin isoforms in the tested groups. It requires further studies in the future. Despite the fact that no statistically important differences was determined in adiponectin concentrations in the blood of population under study, the difference may concern the activity of individual isoforms in the blood between the groups.
So far, only Trinh et al. attempted to analyze the correlation between the adipokines concentration in the blood and fat tissue and CSU development. They analyzed 191 patients with diagnosed CSU, in whom significantly reduced adiponectin concentrations in blood was found, comparing with the control group. Moreover, no correlation between adiponectin concentration and CSU resistance to treatment was observed [32].
Conclusions
In spite of continuously expanded knowledge, CSU etiopathogenesis has not been completely discovered. At the present stage, individual studies relating to the correlation between adipokines concentration and CSU occurrence do not permit to draw unambiguous conclusions about the role they play in CSU pathogenesis. It is necessary to conduct further studies to comprehensively verify and explain the correlation.