Introduction
The intestinal immune system functions in the prevention of the invasion of harmful pathogens while maintaining tolerance of innocuous food substances and commensal microorganisms. The intestinal immune system also shows remarkable heterogeneity along the length of the gut, which reflects regional differences in intestinal function as well as sitespecific intrinsic and environmental factors [1, 2]. The human large intestine, which is composed of the cecum (and associated blind-ended appendix), the ascending, transverse, descending, and sigmoid colon, as well as the rectum, terminates at the anal canal. Microbiota density increases from the upper to the lower gastrointestinal tract until it reaches its peak in the colon, which contains the highest microbial density and load [3]. To maintain local tissue homeostasis, different intestinal segments have adopted unique defense strategies, including alterations in epithelial subset composition and function, mucus layer integrity, and regionalized immune system specialization [1].
The intestinal epithelium and underlying lamina propria (LP) are the major sites of immune cell accumulation within the intestine, and lymphocytes in each of these compartments have unique ontogeny, phenotypes, and functions [4]. Intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) consist mainly of T cells, but innate lymphocytes, including innate lymphoid cells, γδT cells, mucosal-associated invariant T (MAIT) cells, natural killer T (NKT) cells, and natural killer (NK) cells, also reside in the intestine [3, 5]. Therefore, additional research is required to assess variations in the composition of immune cell subsets within different segments of the human intestine under distinct physiological and pathological conditions.
MAIT cells express a semi-invariant T cell receptor (TCR) repertoire, an invariant Vα7.2-Jα33 TCRα chain, and recognize a major histocompatibility complex (MHC) class Ib molecule, MHC-related 1 (MR1) [6, 7]. MAIT cells are found in peripheral tissues, such as the mucosa of the intestine and lung, and are particularly abundant in the liver [8, 9]. MAIT cells are a population of MR1-restricted innate-like T cells that are involved in anti-bacterial immunity and that are activated by cells infected with various strains of bacteria and yeast but not by cells infected with viruses [10]. However, recent studies have demonstrated that MAIT cells are also activated by cytokines following viral infections, such as human immunodeficiency virus and hepatitis C virus [11, 12]. The activation by bacteria requires a cognate interaction between the invariant TCR and MR1, which can present a bacterial-derived ligand. Thus, the development of MAIT cells depends on the presence of microbial flora, and mature MAIT cells are activated in the presence of various bacteria and fungi in an MR1-dependent manner [13, 14]. It was recently reported that MAIT cells accumulate in the inflamed mucosa of patients with inflammatory bowel diseases (IBD), where they display increased cytokine secretion capacities [15-18]. Thus, this large T lymphocyte population is likely to have an important role in the intestinal immune system, but its role and distribution within the large intestine have not been fully elucidated. Therefore, we aimed to analyze the distribution of MAIT cells in different regions of the human large intestine, specifically in the cecum and colon.
Material and methods
Human samples
Heparinized peripheral blood and surgically resected tissues of the cecum and colon were obtained from 4 patients with cecal appendix cancer (CAC) (cecal cancer, n = 3, appendix cancer, n = 1) and 8 patients with colorectal cancer (CRC) (ascending colon cancer, n = 4, transverse colon cancer, n = 1, sigmoid colon cancer, n = 1, rectal cancer, n = 2) at Niigata University Hospital between April 2013 and March 2015. The characteristics of the patients are shown in Table 1. Because we could not separately analyze populations of IELs and LPLs using biopsy samples, we used surgically resected tissues of the cecum and colon that were distant from the tumor in patients with CAC and CRC to obtain both IELs and LPLs. This work was conducted in accordance with the Declaration of Helsinki. Written informed consent under institutional review board-approved protocols (approval no. 1474) at Niigata University Medical and Dental Hospital was appropriately obtained from all the individuals enrolled in this study.
Cell isolation
Peripheral blood lymphocytes (PBL) were obtained by Ficoll-Paque (GE Healthcare Bio-Sciences, Uppsala, Sweden) gradient centrifugation. Surgically removed cecum and colon tissues were collected in sterile Eagle’s minimum essential medium (MEM) (GIBCO/Life Technologies, Grand Island, NY) supplemented with 5 mM HEPES and 10% heat-inactivated fetal calf serum (FCS). The tissues were then cut into pieces in phosphate-buffered saline (PBS) supplemented with 5% FCS. The epithelial layer was removed with 1 mM ethylenediaminetetraacetic acid (EDTA, Sigma-Aldrich, St. Louis, MO, USA) and 1 mM dithiothreitol (Sigma). After continuous agitation for 15 min at 37°C, the single cell suspension was pelleted from supernatant and washed once with RPMI 1640 medium (GIBCO/Life Technologies) supplemented with 10% FCS. IELs were then separated on a Percoll (GE Healthcare Bio-Sciences) density gradient. A discontinuous density gradient (40% and 75%) was used, and cells that layered between 40% and 75% fractions were collected as IELs. To release LPLs, the remaining tissues were treated with Eagle’s MEM (GIBCO/Life Technologies) supplemented with 5 mM HEPES, 0.1% collagenase, and 0.01% Trypsin inhibitor (Sigma Chemical Co.), and then shaken at 37°C for 60 min. The enzymatically digested tissues were pressed through a 200-gauge stainless steel mesh, washed twice, and suspended in MEM containing of 5 mM HEPES and 5% heat-inactivated FCS. Cell suspensions were overlaid on Ficoll-Paque (GE Healthcare Bio-Sciences) and centrifuged at 1500 rpm for 10 min. After centrifugation, cells were washed with PBS (GIBCO/Life Technologies) containing 0.5% heat-inactivated FCS and counted in a hemocytometer; the numbers of viable cells were determined using trypan blue exclusion assays (the viability of all isolated cells was greater than 90%).
Reagents
Monoclonal antibodies (mAb) used for immunofluorescence assay were anti-TCR-γδ-PE (Beckman Coulter, Brea, CA, USA), anti-CD161-FITC, anti-CD3-PerCP, anti-CD8-PerCP, anti-CD69-PE, anti-NKG2D-PE, anti-NKG2A-PerCP, anti-IL-7R-PE, anti-CD195-PE, anti-CD196-PerCP (Becton Dickinson), anti-TCR-Vα7.2-APC (BioLegend, San Diego, CA). Monoclonal antibody used for immune histochemical staining was anti-TCR-Vα7.2 (BioLegend).
Flow cytometry
Cells (105) were labeled with several mAbs at 4°C for 30 min in darkness for the surface antigens, and then washed 2 times and acquired by FACS Calibur flow cytometer (Becton Dickinson). Data were analyzed using Flow Jo software (Tree Star Inc., Ashland, OR) and Cell quest pro (Becton Dickinson). MAIT cells in total blood/colon mononuclear populations were gated based on CD161high Vα7.2+ expressions [9, 14, 19]. The percentage of MAIT cells was calculated as follows: % MAIT (number of CD3+ TCR-γδ- CD161high Vα7.2+ cells)/(number of CD3+TCR-γδ- cells). We used isotypic controls as controls, and 7-amino-actinomycin D was used to gate out dead cells.
Immunohistochemistry
Cryostat sections 8-µm thick were cut and fixed in cold acetone for 5 min. After immersion in blocking serum, sections were incubated with mouse anti-human TCR-Vα7.2 (BioLegend) at a 1 : 100 dilution in PBS supplemented with 3% BSA at 4°C overnight. After successive washing in PBS, sections were incubated with biotinylated anti-mouse immunoglobulin at a 1 : 100 dilution in PBS supplemented with 5% BSA.
Immunohistochemical detection was performed according to the avidin-biotin-peroxidase complex method using the Vectastain Elite ABC kit (Vector Laboratories, Inc., Burlingame, CA). Sections were finally developed with diaminobenzidine substrate (Muto Pure Chemicals, Tokyo, Japan). Specimens were then counterstained with Hematoxylin-eosin and mounted.
Results
MAIT cells are abundant as IELs in the cecum
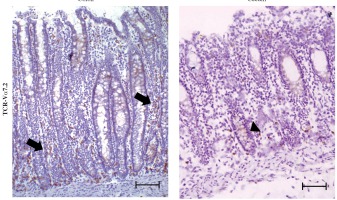
MAIT cells can be identified as CD3+TCR-γδ-CD161hi TCR-Vα7.2+ lymphocytes (Fig. 1A) [9, 14, 19]. Representative results of the identification of MAIT cells in patients with CRC and CAC are shown in Figure 1B. To examine the differences between the cecum and colon, we measured and compared the frequency of MAIT cells among CD3+TCR-γδ- lymphocytes in the cecum of patients with CAC and in the colon of patients with CRC. After a comparison of the frequencies of MAIT cells among populations of IELs and LPLs, the cecum showed a significantly increased frequency of MAIT cells among IELs compared with the colon [0.74 (0.3-2.03) vs. 4.415 (2.23-5.05), p < 0.01] [median (range)] (Fig. 2). However, both the cecum and colon showed similar frequencies of MAIT cells among LPLs (Fig. 2). We further examined the distribution of MAIT cells in the cecum and colon by immunohistochemistry. As shown in Figure 3, we detected TCR-Vα7.2+ cells mainly in the lamina propria of the colon (arrows in Figure 3), but TCR-Vα7.2+ cells were also detected in intraepithelial regions of the cecum (arrowhead in Fig. 3).
Fig. 1
Identification and analysis of mucosal-associated invariant T (MAIT) cells in the blood, colon and cecum of patients. A) Representative results of flow cytometry gating on CD3+TCR-γδ– cells in the peripheral blood and intraepithelial lymphocytes (IELs) in the colon from a patient are shown. B) Representative results of flow cytometry gating on TCR-Vα7.2+CD161high MAIT cells in the peripheral blood, IEL and lamina propria lymphocytes (LPLs) in the colon and cecum from patients are shown. PBL – peripheral blood lymphocytes
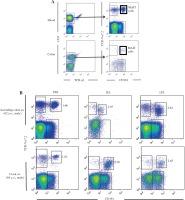
Fig. 2
Comparison of the frequency of mucosal-associated invariant T (MAIT) cells in the peripheral blood, intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) in the colon and cecum. The proportion of MAIT cells in the blood, IELs and LPLs in the colon and cecum of patients with colorectal cancer or cecal appendix cancer was analyzed by flow cytometry. The proportion of MAIT cells among IELs was significantly increased in the cecum compared with that in the colon. The individual data are presented, and bars indicate the means ± standard deviations. *p < 0.01. PBL – peripheral blood lymphocytes
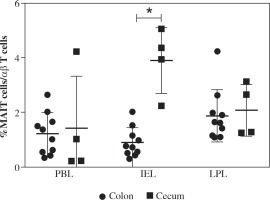
Fig. 3
TCR-Vα7.2+ cells accumulated among the lamina propria lymphocytes (LPLs) in the colon. Surgically resected colon and cecal tissues were obtained from patients with colorectal cancer (CRC) and cecal appendix cancer (CAC), respectively, and the tissues distant from the tumor were stained with anti-TCR-Vα7.2 antibodies. This staining strategy allowed for the identification of TCR-Vα7.2+ cells. Representative results from 8 patients with CRC and 4 patients with CAC are shown. TCR-Vα7.2+ cells accumulated among the LPLs in the colon (arrows) and were also detected in intraepithelial regions in the cecum (arrowhead). Scale bar represents 100 μm (original magnification, ×100)
Phenotypic analysis of MAIT cells in the colon and cecum
We then analyzed the phenotypes of MAIT cells. Although the frequency of MAIT cells among IELs was significantly different from that of LPLs, no significant difference was observed in the frequencies of CD8+ cells, CD69+ cells and natural killer group 2, member D (NKG2D)+ cells among MAIT cells between the cecum and the colon (Fig. 4A-C). However, the expression of CD69 on MAIT cells was significantly increased in the cecum and colon compared with that in the blood (all p values < 0.01) (Fig. 4B). Moreover, compared with the colon, the frequencies of natural killer group 2, member A (NKG2A)+ cells among MAIT cells were significantly increased in the cecum (all p values < 0.01) (Fig. 4D). These results suggest that the MAIT cells were more likely to be activated in the gut, especially in the cecum, than in the blood. We also analyzed the expression levels of C-C chemokine receptor type 5 (CCR5) and CCR6, but no significant differences were detected between the gut and the blood (Fig. 5A,B). However, the expression level of IL-7 receptor (IL-7R) on MAIT cells was significantly decreased in the IELs compared with that in the blood (all p values < 0.05) (Fig. 5C). Therefore, we found that the distribution of MAIT cells was different between the cecum and colon but that the phenotypes of MAIT cells were not so different between the cecum and colon except for the expression of NKG2A.
Fig. 4
Expression of markers associated with the function of mucosal-associated invariant T (MAIT) cells in the blood, colon and cecum. Representative results of flow cytometry gating on TCR-Vα7.2+CD161high MAIT cells in the peripheral blood, intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) in the cecum from a patient are shown (A). Dots indicate the percentages of CD8– (B), CD69– (C), NKG2D– (D) and NKG2A– (E) positive cells among MAIT cells in the blood, colon, and cecum of patients with colorectal cancer (CRC) or cecal appendix cancer (CAC). The expression level of CD69 on MAIT cells among IELs and LPLs was significantly increased compared with that in the blood (C). The expression level of NKG2A on MAIT cells was significantly increased in the blood, IELs, and LPLs of patients with CAC compared with that in patients with CRC (E). The individual data are presented, and bars indicate the means ± standard deviations; *p < 0.01, **p < 0.01 (compared with peripheral blood lymphocytes – PBL)

Fig. 5
Expression of markers associated with the function of mucosal-associated invariant T (MAIT) cells in the blood, colon, and cecum. Representative results of flow cytometry gating on TCR-Vα7.2+CD161high MAIT cells in the peripheral blood, intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) in the cecum from a patient are shown (A). Dots indicate the percentages of CCR5– (B), CCR6– (C), and IL-7R– (D) positive cells among MAIT cells in the blood, colon, and cecum of patients with colorectal cancer or cecal appendix cancer. The expression level of IL-7R on MAIT cells among IELs was significantly decreased compared with that in the blood (D). The individual data are presented, and bars indicate the means ± standard deviations; **p < 0.05 (compared with peripheral blood lymphocytes – PBL)

Discussion
In the present study, we investigated the distribution of MAIT cells in the intestinal epithelium and the underlying lamina propria of the cecum and colon. We found that, compared with the colon, the cecum showed a significantly increased frequency of MAIT cells in the intestinal epithelium. The expression of CD69, which is a marker of activation and tissue residency, on MAIT cells was significantly increased in the cecum and colon compared with that in the blood, and the frequencies of NKG2A+ cells among MAIT cells were significantly increased in the cecum. These results suggest that MAIT cells are more likely to be activated in the intestinal epithelium of the cecum than in the colon and blood.
The intestinal microbiome plays an essential role in the health of the host and in the modulation of local immune cell development, composition, and function [3]. The large intestine contains the highest microbial density and load [1]. In the gut of several mammals, including humans, mice, and rats, microbiota are more associated with the proximal large intestine than the small intestine or the distal large intestine [20]. Moreover, the cecal appendix has especially been shown to have an important interaction with the intestinal flora [21]. Therefore, we considered that the distribution of MAIT cells should be different in the cecum and colon. Interestingly, although ulcerative colitis (UC) is characterized by continuous mucosal inflammation that extends proximally from the rectum without any skip areas, the appearance of inflammation near the appendix in patients with distal UC has been highlighted in recent decades [22]. Such “skip lesions” may also suggest that the cecal appendix might have a different immunological condition, and therefore, the cecal appendix should be investigated differently from the colon.
The intestinal epithelium and underlying LP are the major sites of immune cell accumulation within the intestine, and lymphocytes in each of these compartments have unique ontogeny, phenotypes, and functions [4]. However, previous studies have primarily reported on the characteristics of T cells in populations of IELs and LPLs, and the origin and function of T cells are quite different among IELs and LPLs [4]. Sundstorm et al. recently reported that MAIT cells were significantly accumulated in colon adenocarcinoma tissues and unaffected colon LP and that significantly lower frequencies of interferon-δ-producing MAIT cells were observed in the tumor tissues [23]. Moreover, consistent with the present study, no significant differences in MAIT cell frequencies were noted among LPL between the different locations along the large intestine [23]. In the present study, we found that compared with the colon, the cecum exhibited a significantly increased frequency of MAIT cells in the intestinal epithelium and that the frequency of NKG2A+ cells among MAIT cells was significantly increased in the cecum. NKG2A is one of the important inhibitory NK cell receptors that is expressed not only on NK cells but also on T cells, NKT cells, and MAIT cells. The expression of NKG2A is upregulated after activation [19]. Moreover, MAIT cells are rapidly recruited to sites of inflammation [8]. Therefore, we speculated that the increase in activated MAIT cells might be associated with the accumulation of MAIT cells in the intestinal epithelium of the cecum.
Circulating and tumor-infiltrating MAIT cells have also been reported in patients with CRC [24, 25]. The proportion of circulating memory CD8+ MAIT cells was significantly reduced while the proportion of tumor-infiltrating MAIT cells was increased, especially in patients with advanced CRC [24]. Therefore, MAIT cells may participate in immune surveillance in CRC. However, the increase in tumor-infiltrating MAIT cells was correlated with poor survival of patients with CRC [25]. Interestingly, an important infiltration by IL-17-producing cells is a marker of poor prognosis in CRC [26], and MAIT cells primarily produce tumor necrosis factor α (TNF-α), IL-17, and interferon γ when stimulated in vitro [27, 28]. We also observed that MAIT cells from patients with IBD secrete significantly more TNF-α and IL-17 than those from healthy donors [18]. These findings suggest that IL-17 production may be pro-oncogenic and that sustained infiltration of MAIT cells would increase the risk of cancer development. Further studies are required to confirm this hypothesis and to reveal the mechanisms that are involved in the infiltration and differentiation of MAIT cells in patients with IBD and CRC.
In conclusion, the present study suggests that the distribution of MAIT cells may be different between the human cecum and colon and that more MAIT cells may be activated in the intestinal epithelium of the cecum than in the colon and blood. However, any possible biases in the present study can be enumerated. First, the small number of patients studied, especially in the CAC group, might explain the low statistical power of these data. Second, because we could not separately analyze populations of IELs and LPLs using biopsy samples, we used surgically resected tissues of the cecum and colon that were distant from the tumor in patients with CAC and CRC to obtain both IELs and LPLs. Therefore, the influence of CAC and CRC should be considered. Third, we could not investigate cytokine production by MAIT cells in the cecum and colon after in vitro stimulation because the number of separated MAIT cells was limited. Therefore, further studies are required to clarify the distribution and function of MAIT cells within the human large intestine.


