Introduction
Renal artery aneurysms are rare; their incidence in the adult population is estimated at 0.01–0.09%, i.e. about 20% all of visceral artery aneurysms [1–5]. Typically, they are found incidentally, often during diagnostics of arterial hypertension, and renal aneurysms are more prevalent in these patients. If an aneurysm is asymptomatic and smaller than 20 mm in diameter, it only requires the diagnostic surveillance. Symptomatic and large aneurysms need invasive treatment, with elective surgical repair (tangential excision with primary repair, patch plasty, or bypass) being the preferred modality. In rare cases more complex reconstructions, like extracorporeal reconstruction with autotransplantation, or even nephrectomy are needed. All these procedures are primarily aimed at the prophylaxis of aneurysm rupture, but also facilitate pharmacological management of arterial hypertension, which very often accompanies renal artery disease [1–9].
With the advancement of endovascular techniques and armamentarium, minimally invasive percutaneous endovascular approach, comprising stenting and/or coil embolization, is increasingly used for the management of these visceral aneurysms. However, since most renal artery aneurysms are located at bifurcations of these blood vessels, their endovascular repair remains challenging. Typical endovascular techniques, such as the standard single-stent or double-stents techniques, can result in incomplete coverage of the aneurysm, floating stent struts, or formation of a long metallic neocarina [1–4, 7, 10]. Therefore, techniques that have been developed for the management of lesions located at the bifurcations of coronary arteries – where this problem is also relevant – can be used in these difficult-to-manage arteries [11]. Particularly, the crush technique, which comprises upfront stenting of the side branch with protrusion of the stent into the proximal main branch, followed by stenting of the main branch, can be applied. This technique allows for a full coverage of the side branch ostium, irrespective of the angle between both branches. In this case report, we present endovascular treatment of 2 patients presenting with renal artery aneurysms located at bifurcation, in which we used this particular endovascular technique adopted from the coronary interventions [1–5, 11]. In these patients stenting was augmented with aneurysm embolisation with coils, which were introduced through the stent struts.
Case report
During diagnostics workout for uncontrollable arterial hypertension, we revealed aneurysm of the left renal artery in a 43-year-old female patient. Maximal diameter of this aneurysm was 28 mm, and it was located at the bifurcation of the main renal artery; thus, according to the Rundback classification, it was a type II aneurysm. The angle between the main and side branches was 150o. The side branch was almost as wide as the main one, and its proximal part was also aneurysmatically dilated (Figures 1 A, A1). In this case, considering the angiographic picture, endovascular management using a single stentgraft would result in ischaemia of approximately 50% of the kidney parenchyma. The other possible option would involve implantation of a flow-diverter stent, with possible thrombosis of the aneurysm and without occlusion of the side branch. Still, such a favourable outcome was not guaranteed. Therefore, we decided to address this aneurysm using the crush stent technique, with subsequent embolisation of the aneurysm with coils.
Figure 1
Patient 1. A – left renal artery aneurysm in the anterior-posterior projection; A1 – the scheme of aneurysm and the localisation of renal artery branches; r – renal artery, m – main branch, s – side branch, a – aneurysm; B – guidewires navigated to the distal segment of main branch (arrows point guidewire in the main and side branches; guidewire follows the curvature of aneurysm; arrowhead points guidewire in the main branch); C – successful reposition of guidewire in the side branch (arrow: guidewire in the side branch, prepared for stent introduction; arrowhead: guidewire in the main branch); D – successful navigation of the stent to the side branch (arrowhead), arrow points guidewire in the main branch; E – both stents are introduced and ready for expansion (arrow: stent in the side branch, arrowhead: stent in the main branch); aneurysm in this picture is seen from a different angle, in order to better visualize the stents; F – stent in the side branch has been expanded, it is properly positioned; G – aneurysm filled with coils, good inflow to the main branch (arrowhead); H – aneurysm filled with coils, good inflow to the side branch (arrowhead); guidewire is still inside the side branch (arrow)
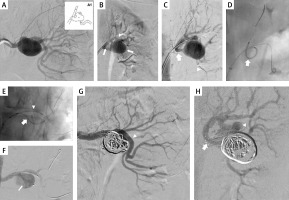
First, we navigated a 6F/45 cm introducer sheath into the proximal part of the renal artery. Then, we introduced two 0.014” guidewires. The first one was introduced to the distal part of the main branch, and the second one – carefully, following the curvature of the aneurysm – to the distal part of the side branch (Figure 1 B). Even if it was possible to introduce the stent to the side branch, the stent was still blocked by the opposite wall of the aneurysm and its repositioning was not feasible. Therefore, we withdrew the stent and introduced a 2.5/20 mm MINI TREK Coronary Dilatation Catheter (Abbott Vascular, Abbott Park, IL, USA), which was inflated to the pressure of 6 atm, which allowed for a favourable reposition of the guidewire (Figure 1 B). Thereafter, over this guidewire, we introduced a 3 × 28 mm Xience stent (Abbott Vascular, Abbott Park, IL, USA) into the side branch, and then a 4 × 38 mm Xience stent to the main branch. The final position of the stents is shown in Figure 1 E. Once the proper position of the stents was obtained, we expanded the stent in the side branch with a balloon at a pressure of 11–20 atm. Then, the stent in the main branch was expanded under a pressure of 10–24 atm. Through the stent struts we introduced a Progreat microcatheter (Terumo, Tokyo, Japan) to the aneurysm, and using this microcatheter we filled its sac with 6 embolisation coils. Control arteriography demonstrated good inflow to the main and side branches, and complete closure of the aneurysm with coils (Figures 1 G, H).
There was a good inflow and complete aneurysm closure demonstrated by CT and digital subtraction angiography at follow-up 18 months after the treatment.
The second patient was a 68-year-old female, who presented with arterial hypertension and during diagnostics workout was diagnosed with 47 mm wide, type II aneurysm of the right renal artery. Similarly to the previous case, this aneurysm was localised at the bifurcation, and the main branch was also as big as the side branch; the angle between these branches was 180o, and the neck of aneurysm was 12 mm long (Figures 2 A, B). Considering the similar angioarchitecture, we decided to address this aneurysm using the crush technique and coils.
Figure 2
Patient 2. A, B – right renal artery aneurysm in 2 projections (arrows: main and side branch); C – guidewires (arrows) navigated to both branches; D – both stents are introduced and ready for expansion (arrow: stent in the main branch, arrowhead: stent in the side branch); E – both stents successfully implanted (arrow: stent in the main branch, arrowhead: stent in the side branch); F – microcatheter (arrow) introduced into the aneurysm; G – aneurysm filled with coils (arrow), good inflow to both branches (arrowheads); H – final result with good inflow to the kidney through both branches
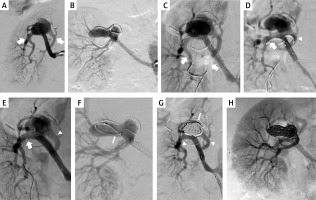
The initial steps of the procedure were similar to the previous patient (Figure 2 C). However, because of a slightly different anatomy, first we introduced the stent (4 × 38 mm Xience) to the main branch, then the second one (3 × 38 mm Xience) to the side branch, and thereafter we modified positions of the stents in order to deploy them according to the crush technique (Figure 2 D). After positioning the stents properly, we expanded the stent in the side branch, and then in the main branch under similar pressures as previously described (Figure 2 E). Then, we filled the aneurysm with 5 coils, using a Rebar microcatheter (Medtronic, Minneapolis, MN, USA). The aneurysm was completely closed with coils (Figures 2 G, H).
There was a good inflow and complete aneurysm closure demonstrated by ultrasonography at follow-ups, which were performed 3, 6, and 16 months after the treatment.
Discussion
Type I and II renal artery aneurysms are usually managed endovascularly by remodeling techniques, using the stents and coils [5, 9, 12, 13]. Still, in a case of the wide side branch originating from the aneurysm, typical stent implantation is not a good option. Such challenging renal aneurysms should rather be managed using a different technique, and the crush stent technique adopted from the coronary interventions seems to be an appropriate solution. We have successfully used this technique in 2 patients requiring invasive treatment of renal aneurysms. Of note, the crush technique in renal artery aneurysms slightly differs from that of a coronary intervention [11]. In the case of renal artery aneurysm, both stents should be simultaneously introduced and thereafter deployed sequentially, without previous rewiring and opening of the struts of the side branch stent. The proximal optimisation technique, typical for coronary procedures, is not used here. The main purpose of stenting of the side branch is to prevent its closure after coil embolisation [5]. It should be emphasised that a proper positioning of the guidewire in the side branch, which is indispensable for stent introduction, may be very difficult. It was especially challenging in Patient 1. Usually, during navigation, the guidewire follows the curvature of aneurysm (Figure 1 B). If the positioning of guidewire is not optimal, it can be repositioned using the anchoring technique. This technique consists of the introduction of balloon to the side branch, and its inflation, which stabilises the guidewire. Thereafter, a gentle straightening and reposition of the guidewire alongside the target vessel is feasible (Figure 1 C). Alternatively, a self-expanding stent could be used. After partial deployment of its distal part, such a stent becomes stabilised, and its subsequent reposition can be performed. However, the use of a self-expanding stent is associated with a risk of its uncontrollable migration to the aneurysmal sac, where neither reposition nor redeployment (except for self-expanding stent dedicated for cerebral arteries, which are typically not used here because of their high cost) becomes possible [5, 7, 8, 10].
In conclusion, the crush technique seems to be an adequate technique for the management of renal artery aneurysms located at bifurcations of these arteries.








