Introduction
Epidemiologic studies indicate that the universal frequency of allergic rhinitis (AR) is growing and affecting 400 million people [1]. AR is described as “indications of sneezing, nasal pruritus, airflow congestion, and mostly clear nasal discharge induced by IgE-mediated reactions against inhaled allergens”, and includes mucosal swelling driven by type 2 helper T (Th2) cells [2].
Previously, it was recognized that acidic mammalian chitinase (AMCase) is a critical element of IL-13-stimulated reaction in Th2-dominated disorder in both human and animal asthma models [3-5]. Furthermore, it was thought that chitinase from house dust mite, such as Dermatophagoides pteronyssinus (D. pteronyssinus) and Dermatophagoides farinae (D. farinae), present a high potential of binding to IgE in atopic human sera [6], and are the primary allergens for humans and dogs with house dust mite hypersensitivity [7]. The production of cytokines and chemokines, such as IL-8, which is a member of the CXC chemokine family, is heightened by the airway epithelial cells in response to environmental elements, including pollens, cigarette smoke, air contaminations, and microorganisms with their secreted extracellular proteins [8-10]. IL-8 has been considered a crucial factor in airway inflammatory disorder [11, 12]. Moreover, high levels of IL-8 have been detected in the bronchoalveolar lavage fluid of patients with airway swelling disorders [13].
Therefore, the present study was undertaken to:
Investigate the human serum expression levels of AMCase and IL-8 in patients with moderate/severe persistent allergic rhinitis (M/S PAR) compared to patients with mild persistent allergic rhinitis (M PAR) and the control group.
Evaluate mRNA expression of AMCase and IL-8 in human inferior turbinate mucosa in patients with M/S PAR compared to its expression in patients with M PAR and the control group.
Determine the correlation between serum levels of AMCase and IL-8 as well as the correlation of each serum levels with various pathological parameters.
Material and methods
Subjects
Patients were administered into three groups: M/S PAR (n = 30), M PAR (n = 30), and control (n = 30) groups. The selected patients presented with no history of nasal infection, allergy, smoking, and ongoing drug treatment. During surgery, inferior turbinate mucosal biopsies were obtained from all included patients. The diagnosis of AR was established on the basis of medical history and symptoms (i.e., sneezing, rhinorrhea, and nasal congestion occurring most days) lasting for at least 2 years, positive skin test results, and positive specific IgE ELISA test for house dust mite (Astra Biotech GmbH, Berlin, Germany). AR patients were classified as mild or moderate/severe persistent according to allergic rhinitis and its impact on asthma criteria [14]. Exclusion criteria consisted of current smoking status, medical history of asthma and recurrent infections of respiratory tract, and the use of anti-allergic drugs within 4 weeks prior the study. Before tissue specimens were obtained, all the protocols have been approved by the institutional review board at our institution, and all participants have signed a written informed consent. Venous blood samples were obtained from patients. Blood drawing, handling, and storage were performed by the same researchers. Some of the tissues were immediately frozen in liquid nitrogen and stored at –70 centigrade for the extraction of RNA, and some samples were paraphined for hematoxylin and eosin (H&E) staining. Four-millimeter punch nasal biopsies were collected from inferior turbinate of both patients and control groups. Biopsy specimens were submerged immediately in 10% buffered formalin and prepared for paraffin embedding. Paraffin blocks were cut into sections of four microns thick, which were mounted on glass slides. They were prepared for routine H&E staining for conventional histopathology (Table 1).
Table 1
Sequences of PCR primers
Real-time polymerase chain reaction analysis of AMCase and IL-8
The total RNA was isolated from nasal tissue using Biozol reagent (BioFlux, Japan). The quantity and quality of RNAs were measured by spectrophotometry using wavelength absorption ratio (260/280). Some RNA samples (1 mg/ml) were coupled with DNase1(10 u/ml) for 30 min to avoid DNA contamination. Following the second RNA extraction, cDNA was produced in a 20 ml reaction tube from 1 mg total RNA (Thermo Fisher Scientific, Vilnius, Lithuania) with a random primer. The reactions were incubated at 25°C for 5 min, 42°C for 1 h, and 72°C for 5 min. The primer sequences, which were used for each gene included in the study are shown in Table 1. β-actin was used as the internal control. Expressions were determined by quantitative real-time polymerase chain reaction (PCR) using SYBR Green PCR master mix (Takara, Kyoto, Japan). A standard curve was established, and efficiency was determined for each set of primers. The determined efficiency was typically above 90%. The data were expressed as fold changes, using comparative cycle threshold (ΔΔct method). Relative gene expression was defined by the 2-ΔΔct method.
Assessment of AMCase and IL-8 levels by ELISA
AMCase and IL-8 levels in serum of patients with M/S PAR, patients with M PAR, and controls were measured using ELISA as per the manufacturer’s protocol (Abnova, Taipei, Taiwan). Data were expressed as ng/ml protein. Protein concentration was determined with the Starsat ELISA reader (Awarness, Phoenix, AZ, USA).
Statistical analyses
Statistical analyses were carried out using SPSS for Windows (IBM SPSS Statistics, IBM). One-way ANOVA, along with post-hoc Tukey’s test was performed in order to evaluate whether the values obtained by real-time PCR and ELISA differ among the nasal mucosa of healthy controls and patients with mild and M/S PAR. Student’s t-test was used to analyze the differences in specific IgE between M/S PAR and mild PAR. Correlations were assessed with Pearson’s correlation test. A value of p < 0.05 was considered as statistically significant.
Results
Clinical characteristics of participants
The characteristics of patients with persistent allergic rhinitis and healthy controls are demonstrated in Table 2. As shown in Table 2, age was similarly distributed among the healthy controls and the patients with PAR. The duration of disease did not significantly differ between the groups of patients with M and M/S PAR, and serum total IgE levels were higher in patients with M and M/S PAR than in individuals with healthy nasal mucosa.
Table 2
Demographic characteristics of participants
Gene expression levels of AMCase and IL-8 in nasal mucosa
The gene expression levels of AMCase and IL-8 were determined in healthy, M, and M/S PAR nasal mucosa samples using real-time PCR. As shown in Figure 1 and Table 3, the expression levels of AMCase and IL-8 presented significant increase in nasal mucosa of patients with M/S PAR compared to nasal mucosa of patients with M PAR and the controls. Similarly, the gene expressions of AMCase and IL-8 were statistically different between M PAR and control groups.
Fig. 1
Real-time PCR analysis of AMCase (A) and IL-8 (B). mRNA in inferior turbinate nasal mucosal tissue from controls and patients with M PAR and M/S PAR. * indicates significant difference in the expression levels of AMCase and IL-8 among normal, mild, and moderate/severe persistent allergic nasal mucosa (p < 0.05). Results are shown as mean ±SD (Table 3)
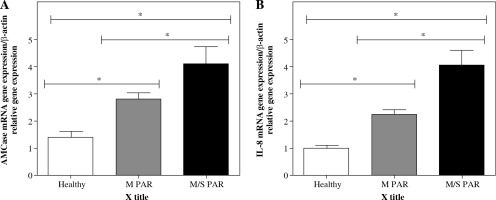
Table 3
Comparison of baseline characteristics, AMCase gene expression, IL-8 gene expression, serum levels of AMCase, and IL-8 among control group, patients with M PAR, and patients with M/S PAR
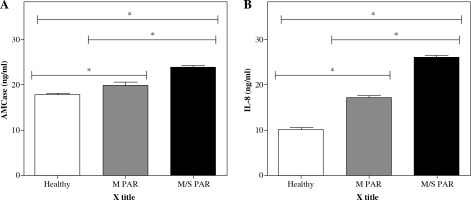
Serum levels of AMCase and IL-8 in patients with M/S PAR, M PAR, and healthy controls
As shown in Figure 2 and Table 3, AMCase and IL-8 levels (ng/ml) were measured in the serum obtained from patients with M/S PAR, M PAR, and healthy controls. Significantly higher levels of AMCase and IL-8 were found in serum obtained from patients with M/S PAR compared to healthy participants (p < 0.05) and patients with M PAR. Likewise, the differences between the patients with M PAR and healthy controls in terms of levels of AMCase and IL-8 were statistically significant (p < 0.05).
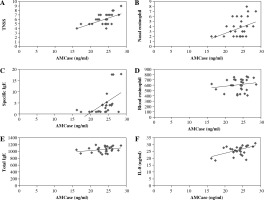
Associations between serum levels of AMCase with immunopathogenesis of M/S PAR
As shown in Figure 3, there were considerable associations between serum AMCase levels and TNSS (R = 0.576, p < 0.05; Fig. 3A), degree of eosinophil infiltration to nasal (R = 0.393, p < 0.05; Fig. 3B), and SIgE (R = 0.524, p < 0.05; Fig. 3C). On the other hand, serum AMCase levels did not correlate with blood eosinophil counts (R = 0.178, p > 0.05; Fig. 3D) or TIgE (R = 0.277, p > 0.05; Fig. 3E). Moreover, the correlation between serum AMCase levels and serum IL-8 levels was statistically significant (R = 0.525, p < 0.05; Fig. 3F).
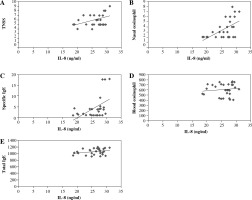
Associations between serum levels of IL-8 with immunopathogenesis of M/S PAR
As shown in Figure 4, the correlations between serum IL-8 levels and TNSS (R = 0.428, p < 0.05; Fig. 4A), degree of eosinophil infiltration to nasal (R = 0.547, p < 0.05; Fig. 4B), and SIgE (R = 0.473, p < 0.05; Fig. 4C) were statistically visible. In contrast, there was no association between serum IL-8 levels with blood eosinophil counts (R = 0.114, p > 0.05; Fig. 4D) and TIgE (R = 0.267, p > 0.05; Fig. 4E).
Discussion
In this study, gene expressions of AMCase and IL-8 in the human nasal mucosa of inferior turbinate as well as the serum levels were investigated. Although local and serum production of AMCase and IL-8 in AR have previously been reported [15-18], this was the first study (as far as the authors of the present study are concerned) conducted on the correlation among AMCase and IL-8 with disease parameters, including disease severity and clinical characteristics. The findings of the present study showed that there was an observable increase in serum levels and gene expression of AMCase and IL-8 in patients with M/S PAR compared to patients with M PAR and the controls. Similar to the data presented here, Cho et al. [15] reported that production of AMCase increased in patients with AR compared to healthy controls. Moreover, Gosset et al. [19] stated that production of IL-8 in patients with AR raised compared to healthy controls. On the contrary, the results obtained from the present study was rejected by Dassonville [20] and Dasilva [21]. Data on cellular spreading is required to disclose practical characteristics of AMCase. The gene expression of AMCase has been recognized by northern blotting analysis in human submandibular gland and stomach [22]. This pattern of spreading suggested that AMCase takes on a significant role in the digestion of ingested chitin-coated animals and plants rather than being a mere defense system [22]. Nevertheless, it is possible that AMCase participates in defense against chitin coating pathogens, such as fungi. Overdijk et al. [23] suggested that the attendance of chitinase in human serum may be due to a function of AMCase in the defense against chitinous pathogens, such as fungi. This suggestion is supported by the observation that a systemic infection with Aspergillus Fumigates results in increasing chitinase levels and this, in turn, can be inhibited by antifungal treatment [24]. However, a great number of macromolecules, consisting of mucine and antibacterial substances, are secreted by submucosal glands and epithelial cells in nasal mucosa. Cho et al. [15] reported that AMCase was noticeably produced in the epithelial cells and submucosal glands in healthy nasal mucosa, indicating its participation in defense against chitin-containing pathogens. Moreover, it may play a role in allergic reaction similar to other inflammatory mediators in allergic rhinitis, due to its up-regulation of allergic conditions. One of the strengths of this study was that the correlations among AMCase and IL-8 with clinical characteristics were analyzed. It was found that serum levels of AMCase and IL-8 were positively associated with SIgE and the number of infiltrating eosinophils to nasal mucosa. Nasal eosinophils could induce inflammation, which cause nasal hyperactivity. Interestingly, there were visible correlations between AMCase and IL-8 with TNSS; therefore, the present data support the concept that AMCase and IL-8 are associated with disease severity. Serum levels of AMCase are associated with congestion and sneezing. In the present study, there was a correlation between IL-8 and sneezing; however, the correlations between both AMCase and IL-8 with rhinorrhea were not statistically significant. In addition, there was a positive correlation between serum levels of AMCase and IL-8, indicating that up-regulation of AMCase was associated with up-regulation of IL-8. Another noteworthy finding was that serum levels of AMCase and IL-8 were not correlated with blood eosinophil count, suggesting that it was more reasonable to estimate the level of eosinophils in tissue than in blood.