Introduction
Atrial fibrillation
Atrial fibrillation (AF) is the most common arrhythmia in the world [1]. Patients with AF require stroke prevention treatment due to increased risk of thromboembolic events [1–3]. Standard treatment involves oral anticoagulation (OAC). However, for patients in whom OAC treatment is contraindicated, surgical stroke prevention can be established. One such procedure is left atrial appendage occlusion (LAAO).
Percutaneous interventions in left atrial appendage management
In recent years, percutaneous interventions have emerged as pivotal approaches in managing cardiovascular conditions, offering minimally invasive alternatives to traditional surgical methods [2]. Several closure methods have been developed, both endocardial and epicardial [4]. Left atrial appendage occlusion proved to be reliable stroke-preventing treatment [2]. However, for all percutaneous interventions topographical information of patients’ anatomical variability plays a crucial role in the outcome of the procedure. Insufficient visualization of organs’ structure can cause damage inflicted with devices introduced into vessels.
Fusion imaging in transcatheter cardiac procedure
In the realm of transcatheter procedures, the convergence of medical imaging technologies has ushered in a paradigm shift, elevating the precision and efficacy of cardiovascular procedures to unprecedented heights. The concept of fusion imaging (FI) has emerged as a pivotal strategy, unifying transesophageal echocardiography (TEE) and fluoroscopy into a singular, synergistic toolset. This dynamic synergy empowers clinicians with real-time, multi-dimensional insights, amalgamating the exquisite anatomical detail of TEE with the fluoroscopic visualization of catheter movement and device deployment [5]. By fusing these complementary modalities, operators gain a comprehensive understanding of cardiac anatomy, device positioning, and procedural progress, thereby ushering in a new era of enhanced patient outcomes and procedural excellence.
Separate imaging downsides
Fluoroscopy allows for the visualization of catheters and blood flow if contrast is used. However, the imaging of heart muscle is severely limited by the amount of contrast medium possible to use [3]. TEE can accurately show the heart, but catheters and other devices are poorly visible [5]. Combining the two images from two displays is crucial for the outcome of the procedure yet involves great experience and effort from the interventionalist.
Aim
The aim of our study was to present, for the first time in Poland, the application of the fusion imaging system in LAAO procedures, while also exploring the crucial role of this system in guiding LAAO interventions, underscoring its advantages, influence on procedural outcomes, and its potential to revolutionize the field of cardiovascular interventions.
Material and methods
Study design
A single-center retrospective study was performed including patients who underwent an LAAO procedure between March 2015 and December 2018. During all procedures FI was used. The most common indications for LAAO procedure were bleeding from gastrointestinal tract (57.8%; n = 48) and stroke despite antithrombotic treatment (25.3%; n = 21). Detailed indications for the LAAO procedure in included patients can be found in Table I.
Table I
Baseline patient characteristics (n = 83)
Procedure
The LAAO procedure in detail was described in our previous papers. Briefly, the LAAO procedure entails percutaneous closure of the left atrial appendage (LAA) using the Amplatzer Amulet or the LAmbre endocardial occluders. After patient heparinization, a catheter is introduced through the femoral vein into the right atrium. Subsequently, a transseptal puncture is performed, and the occluder is positioned within the left atrial appendage.
Fusion imaging
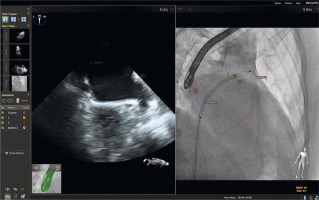
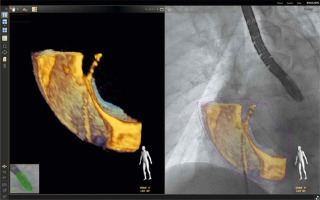
As described by Balzer et al., the EchoNavigator Release II (Philips, Best, Netherlands) system is a multimodal approach that synchronizes live echocardiography and fluoroscopy images in real time [6]. A calibration algorithm tracks the movement of the TEE probe using fluoroscopy. This is achieved through an image-based TEE probe localization algorithm and calibration procedure. After synchronization, the echocardiographic images from TEE automatically follow the motion of the fluoroscopic C-arm. The results of the coregistration process are displayed in a form that allows simultaneous visualization of an X-ray image and up to three echocardiographic views. The X-ray view shows the actual fluoroscopic image. The probe must be centered in this view to allow accurate coregistration of the probe TEE. If this is successful, the probe is displayed with a green outline; if not, e.g. after significant movement of the TEE probe, the probe is displayed with a red outline. The echo view shows images that can only be processed by the echocardiologist.
In the C-arm view, the beam path (i.e., the echocone) of the matrix array transducer is shown as a purple sector corresponding to the position of the TEE probe. Changes in the angulation, rotation, or position of the TEE probe are automatically displayed in this view. The free view displays 2D and 3D information that can be rotated, cropped, zoomed, or segmented by the operator using a sterile cover. Multiplanar reconstruction (MPR) software provides tools for 3D volume segmentation along the three axes (x, y, z) in real time or during post-processing, as well as quantitative analysis. Specific points of interest can be marked and are immediately displayed on the fluoroscopic image. These markers then serve as target points for guiding catheter manipulations. Intraoperative images of FI during LAAO are presented in Figures 1 and 2.
Outcome parameters
In order to assess safety and efficiency of the procedure several parameters were measured: duration of the procedure, amount of contrast medium used, creatinine level after the procedure, overall days spent in hospital after the procedure. All adverse effects caused by LAAO were reported. A follow-up was conducted after 3 and 6 months involving physical examination with echocardiography. Two patients were lost to the first follow-up and another one was lost to the second follow-up.
Statistical analysis
Collected data were tested for normality using the Shapiro-Wilk test, tabulated, and reported as means with standard deviations or numbers and percentages. Statistical analyses were performed using IBM SPSS Statistics (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, version 29.0. Armonk, NY: IBM Corp).
Results
Study population
Our study was conducted on 83 patients (43.4% female; n = 36) with mean age of 72.1 ±8.4. Mean stroke and bleeding risk scores were calculated: CHA2DS2-VASc score: 5.0 ±1.5 and HAS-BLED score: 4.4 ±0.9.
Procedural data
All LAAO procedures were successful (n = 83). Mean total procedure time was 54.9 ±34.3 min. The amount of contrast medium was 33.7 ±22.7 ml. Mean creatinine level after the procedure was 1 ±0.5 mg/dl. On average patients were discharged 4.2 ±3.4 days after the procedure. In 90.4% of patients (n = 75) no procedural adverse effects were reported. Bleeding from the femoral vein occurred in 4 (4.8%) patients, in 3 (3.6%) patients there was cardiac tamponade and 1 (1.2%) patient died on the fourth day after surgery.
Follow-up
A 3-month follow-up did not show any pathologies in 74 (91.4%) patients, with 2 (2.5%) patients having thrombi related to the occluding device. A 6-month follow-up did not reveal any adverse effects in 76 (97.4%) patients. Comprehensive follow-up data are presented in Table II.
Table II
Complications and follow-up outcomes
Discussion
This study is the first report on use of IF in LAAO. In our experience this method is easy to implement and provides better and more intuitive information on localization of the devices and local anatomy.
Over a decade after its inaugural application, LAAO continues to bring about a transformative shift in the approach to mitigating stroke risk among patients afflicted with nonvalvular AF [7]. Numerous investigations have demonstrated favorable short- and mid-term outcomes associated with the LAAO procedure, even among patients at elevated risk [4, 8]. Its adoption has evolved into a global norm. In 2020, the European Society of Cardiology guidelines endorsed LAAO as a viable alternative for AF patients unable to undergo OAC therapy [2]. Currently, LAAO has become a standard procedure performed in hybrid operating rooms or catheterization laboratories[2]. Despite the procedure’s prevalence, LAAO itself is technically demanding, requiring the operator’s precise mastery of cardiac structural anatomy, while the LAA itself exhibits diverse anatomy and delicate, thin-walled musculature [9].
Apart from occluding methods there are epicardial and endocardial left atrial appendage closure techniques [4], [8, 10]. Regardless of method used, safety and effectiveness of the procedure strongly rely on presurgical imaging showing the local topography, which is common for all types of cardiac surgery [11, 12]. What is also important during left atrial appendage procedures is its anatomy regarding differences in structure of the LAA in patients with atrial fibrillation [9, 13]. In patients with AF the LAA is significantly larger but the precise mechanism of remodeling is yet to be discovered [9].
Recently fusion image technique has proven to be an important tool in percutaneous procedures. It is utilized in treatment of structural and congenital heart defects [14–16]. Merging views from fluoroscopy and TEE provides complex information about heart structure and device localization allowing for better execution of the procedure [17].
Growing experience with fusion imaging confirms that it is a safe procedure in terms of mortality [18] and adverse effects [5, 17]. Considering adverse effects supports FI to be a safe and effective method.
Several papers state that FI contributes to lowering total procedure time and amount of contrast medium used. In terms of time our data are comparable with other research, but in our procedures considerably less contrast was used [5, 17]. Minimizing usage of contrast medium can be beneficial to patients suffering from renal failure who need LAAO. It is also possible to reduce the number of renal adverse effects related to percutaneous procedures. Creatinine levels of our patients suggest that the procedure inflicted low damage on renal function.
More safety matters can be brought up involving FI as it is stated that it can diminish the radiation dose given to the patient [19]. Lowering radiation exposure increases the safety of both patients and medical staff. For patients who need multiple percutaneous interventions in a short amount of time those benefits can be especially crucial and can greatly influence treatment success.
Fusion imaging is a technique successfully used in percutaneous heart procedures, especially in LAAO. Combining X-ray with ultrasound reduces the disadvantages of separate methods and provides a more precise view of heart structures allowing for better and faster treatment. FI decreases the duration of the procedure and the amount of contrast medium administered to the patient. Further comparative research should provide more advantages of this method.
Conclusions
The method of fusion imaging is believed to be better than the classical imaging method. It does not carry any additional risks for patients. Moreover, due to the possibility to reduce the radiation dose, it can be more beneficial. We recommend IF to be the standard visualization technique for LAAO procedures.