Introduction
Inducing angiogenesis is a hallmark of cancer and is one of the most investigated pathways in drug discovery [1, 2]. In metastatic colorectal cancer (mCRC), angiogenesis is induced as a response to hypoxia and also to facilitate invasion and dissemination processes [3, 4]. Disruption of angiogenesis by monoclonal antibodies such as the anti-vascular endothelial growth factor (VEGF) bevacizumab has yielded promising clinical activity in several solid cancers [5]. The combination of several chemotherapeutic regimens with bevacizumab in the metastatic setting of CRC was approved worldwide based on various positive randomized and controlled trials [6]. Tumor sidedness in CRC has recently emerged as a promising predictive factor of survival outcomes and therapy response as suggested by recent meta-analyses [7–9]. Recent advances on the clinical relevance of tumor location in mCRC suggest that patients with right-sided tumors have an improved response to first-line bevacizumab [7, 10]. However, other recent reports suggest that left-sided mCRC patients may also benefit more from the combination of chemotherapy with bevacizumab [11, 12].
The treatment of mCRC in low- and middle-income countries is challenging. In Eastern Morocco, where this digestive cancer is the third in terms of incidence in both sexes [13], the significant efforts of the Lalla Salma Foundation and the Ministry of Health have enabled the introduction of several expensive targeted agents for our local setting including bevacizumab for patients covered by public health insurance. In this real-world study, we evaluated the efficacy and the toxicity profile of the combination of bevacizumab with various chemotherapeutic regimens in mCRC. Moreover, we also looked at the benefits of using this antiangiogenic drug on liver metastasectomy in a selected patient group and tested the hypothesis of tumor sidedness as a biomarker of outcomes in mCRC patients treated with bevacizumab in association with chemotherapy. To the best of our knowledge, this is the first paper to report real-world experience of using bevacizumab in mCRC in Morocco.
Material and methods
We retrospectively retrieved archived printed files of patients with mCRC at the Hassan II Regional Cancer Center (Oujda, Morocco) who were treated between 1st January 2014 and 31st December 2019. Access to patients’ data was approved by the local committee of the hospital. Written and informed patients’ consent and ethical committee approval were not needed given the retrospective nature of this report. We performed data collection and storage with complete respect for anonymity and patient data protection. The study was in accordance with the Declaration of Helsinki and its amendments. For transparency and post-publication peer-review, patients’ data are available through a request to the corresponding author. Included patients should have a colorectal adenocarcinoma confirmed by a histopathological examination, assessable data for tumor sidedness, a metastatic disease, and being treated by a combination of first-line bevacizumab with chemotherapy and had continued their treatment with bevacizumab as maintenance in association with fluoropyrimidine or with second-line chemotherapy after disease progression. Left-sided tumors were defined as tumors located in the splenic flexure, rectum, sigmoid colon, and descending colon while tumors located from the cecum to the hepatic flexure were categorized as right-sided. Patients’ follow-up was performed based on clinical examination every three or four weeks and every three months with the aid of computed tomography (CT) scan, carcinoembryonic antigen, and guided by reported symptoms as recommended by international guidelines. Progression-free survival (PFS) was measured from the date of bevacizumab start to the date of progression, last visit, or death. Moreover, overall survival (OS) was measured from the date of mCRC diagnosis to the last event recorded in the patients’ files. First-line bevacizumab was given at a dose of 5 mg/kg every two weeks (q2w) or 7.5 mg/kg every three weeks (3 wk) in association with chemotherapy. Patients who were managed with chemotherapy alone or with other treatment regimens were excluded. Firstly, descriptive summary statistics such as means, their standard deviation, and percentages were used to describe the features of the study population after verifying the normality of data distribution. χ2 or Fisher’s exact test and Student t-test were used to compare the patients’ data according to tumor sidedness. The association of tumor sidedness and other covariates with OS was first investigated using a univariate log-rank test and assessed by Kaplan-Meier survival analysis. A multivariable Cox proportional hazard model was used to study the independent effect of each factor on OS adjusted for covariates. A p-value < 5% was considered significant. All analyses were performed using SPSS 26.0 software (IBM Corp. SPSS Inc., Chicago, IL).
Results
Baseline characteristics of enrolled patients and reported toxicities
A total of 98 CRC patients with adenocarcinoma histology, metastatic disease, and treated with bevacizumab in combination with chemotherapy were included in the analysis (Table 1). The mean age of patients was 52.65 (± 13.662 SD; range: 20–81) and 58% of them were female. Young (≤ 45 years) and elderly patients (≥ 70 years) represented 28.6% and 9.2% of included participants, respectively. Left-sided tumors were the most common in the population (75.5% vs. 24.5%) presenting with cancers in the left colon (38.8) and the rectum (36.7%). RAS mutational profiling and other molecular features were rarely available in our department. Four patients had wild type RAS status. Most patients were physically well and only 28.6% had comorbid conditions including smoking (10.2%), diabetes (6.1%), hypertension (5.1%), and 4.1% had heart disease. Lymph node involvement was seen in 29.6% of patients and was significantly associated with tumor sidedness (p = 0.035) (Table 2). The liver was the principal common metastatic site (55%), which was significantly higher in patients with left-sided tumors (p = 0.013), followed by peritoneal carcinomatosis (45%), lungs (28%), and other unusual locations (6% for all). A single metastatic organ location was the most frequent in our population (48%), followed by patients with two locations (38.8%), and those with more than two locations (11.2%). Surgery was performed for 20 patients, encompassing resection of the primary tumor (14%), hepatic metastasectomy (11%), diverting colostomy (7%), and debulking (3%). Regarding first-line chemotherapeutic combinatorial regimens with bevacizumab, oxaliplatin-based chemotherapy was the dominant choice (88.8%). The remaining group (11.2%) received irinotecan-based protocols. Similarly, second-line chemotherapeutic protocols in combination with bevacizumab used after progression were used for 35 patients based on oxaliplatin-based chemotherapy (85.7%) and irinotecan-based chemotherapy (14.3). Recorded post-bevacizumab toxicities were noted in 28.6% of the study population. This includes manageable and tolerable adverse events including mainly grade I–II proteinuria (10%), bleeding events (10%), thromboembolic events (9%), hypertension (3%), and other rare events such as delayed healing of the stoma, scar dehiscence, and heart failure deterioration. Only one patient had intestinal perforation, which required a successful surgical intervention.
Table 1
Clinicopathologic characteristics and toxicity profile of the study population
Table 2
Patients’ characteristics and their association with tumor sidedness
Survival outcomes
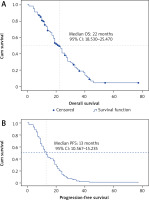
Median OS and PFS in the whole metastatic CRC cohort treated with chemotherapy and bevacizumab were 22 (95% CI: 18.35–25.47) and 13 months (95% CI: 10.567–15.235) respectively (Fig. 1). Improved median OS was noted in young patients (≤ 45 years; 24 months) as compared to those with an age ranging from 46 to 69 years (20 months) and elderly patients (18 months) (log rank p = 0.765). For CRC patients who benefited from liver metastasectomy (n = 11) after bevacizumab use, median OS was 31 months (95% CI: 28.108–33.92) as compared to 14 months for the matched cohort with hepatic non-resectable metastasis (95% CI: 4.135–23.865) (log rank p = 0.204).
Fig. 1
Overall survival (A) and progression-free survival (B) of the whole population treated with bevacizumab-based chemotherapy
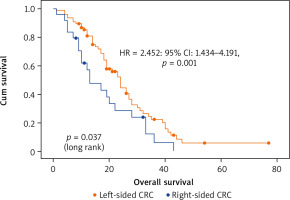
The investigation of potential predictors of OS based on Kaplan-Meier, univariate and multivariate Cox regression showed that tumor sidedness, surgery, number of metastatic locations, and number of bevacizumab administrations were significantly associated with outcomes (Table 3). As expected, we confirmed the prognostic value of tumor sidedness on OS. On univariate analysis, patients with left-sided CRC had significantly superior median OS (24 vs. 13 months; HR = 1.696; 95% CI: 1.018–2.824, p = 0.042). Notably, this observation was confirmed on multivariate analysis after adjustment for covariates (HR = 2.452; 95% CI: 1.434–4.191, p = 0.001) (Fig. 2). Moreover, the number of metastatic locations was also associated with OS (log rank p = 0.03). Patients with three or more metastatic locations had the worst survival as compared to those with 1 or 2 (18 vs. 23 and 24 months respectively; HR = 1.525; 95% CI: 1.084–2.147, p < 0.015). This was further validated on multivariate analysis, which demonstrated a highly significant association of this predictor with OS (HR = 1.94; 95% CI: 1.346–2.800, p < 0.0001). The impact of surgery including metastasectomy, primary tumor resection, and other surgical interventions on patients’ survival was also confirmed in this cohort with a higher median OS that reached 31 months as compared to subjects who did not benefit from any surgery (19 months) (HR = 0.534; 95% CI: 0.321–0.887, p = 0.015). However, this observation was not confirmed on multivariate analysis (HR = 0.804; p = 0.440). Remarkably, after selection of a group of patients treated with primary tumor resection (n = 14) and a matched cohort with no resection, median OS was significantly improved (30 vs. 9 months respectively, log rank p = 0.003) (Fig. 3). Expectedly, a significantly improved OS was markedly noted for patients who received administrations of bevacizumab during the course of their treatment as a combination with first- or second-line chemotherapy and as a maintenance (Fig. 4). Median OS for patients who received between 10 and 20 or more than 20 bevacizumab administrations was 24 and 33 months respectively as compared to those who received fewer than 10 cures (log rank p < 0.0001). Importantly, this was further demonstrated on both univariate (HR = 0.562; 95% CI: 0.417–0.756, p < 0.0001) and multivariate (HR = 0.518; 95% CI: 0.374–0.717, p < 0.0001) Cox regression, which showed a positive impact of bevacizumab use on OS.
Fig. 3
Overall survival of a cohort of metastatic colorectal cancer patients treated with bevacizumab-based chemotherapy who benefited from a primary tumor resection
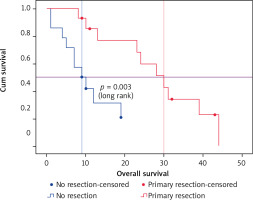
Fig. 4
Overall survival of the whole population treated with bevacizumab according to exposure duration
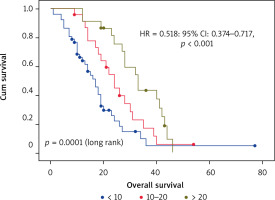
Table 3
Univariate and multivariate analyses of clinicopathological characteristics as predictors of overall survival
Discussion
This real-life study showed that bevacizumab combined with chemotherapy in this setting is a well-tolerated and effective approach as suggested by phase III trials and international guidelines. It also demonstrated that tumor sidedness is a predictor of OS. To our knowledge, our study is one of the few investigations that have reported outcomes of this association for patients with mCRC in real-world practice and the first in Morocco.
Angiogenesis is routinely targeted in clinical practice for treating various cancers such as CRC and ovarian cancer. In a previous Cochrane systematic review, the addition of the antiangiogenic drug bevacizumab to chemotherapy in mCRC was found to prolong both OS and PFS in the first- and second-line settings [14]. This was confirmed by other recent systematic reviews and meta-analyses suggesting that bevacizumab-based regimens resulted in a significant effect on survival outcomes and response in mCRC [15, 16]. In our study, median cumulative PFS was 13 months, which is similar to that reported by several real-practice reports that showed a 9 to 17 months gain over chemotherapy alone (0.5–6.5 months) [17]. According to a recent systematic review of real-world bevacizumab use in this setting [17], our reported median OS of 22 months broadly corresponds to that found in similar retrospective studies (19–32.4 months) and also landmark randomized and controlled phase III first-line trials [18, 19]. When comparing first-line therapies combined with bevacizumab, the group of patients treated with irinotecan-based chemotherapy had better median OS as compared to the oxaliplatin group (24 vs. 21 months respectively). However, these findings were not statistically significant and are consistent with Kawai’s recent meta-analysis that demonstrated that these two regimens are similar in terms of OS [20]. Notably, we found that patients who received more administrations of bevacizumab experienced longer OS. This may be explained by their higher exposure to this antiangiogenic agent. This is further supported by a recent study that found that survival in mCRC is proportional to the magnitude of bevacizumab exposure [21]. Moreover, our study also found a reduced median OS with increasing age. Clinical activity was noted in younger and also in elderly patients. This last category is considerably under-represented in clinical trials. A previous analysis of the Bevacizumab Regimens’ Investigation of Treatment Effects observational cohort study that assessed the efficacy of bevacizumab-based first-line chemotherapy for mCRC showed that elderly patients had reduced OS but also benefit from this combination [22]. Our study observed a more favorable OS in mCRC patients with liver metastases who benefited from surgical resection after preoperative administration of bevacizumab. Despite the statistically non-significant result that may be due to the small sample size of this group of patients, this is in line with the findings of a recent systematic review that pooled data from observational cohort studies [23]. In fact, the authors demonstrated that using bevacizumab increases the rates of metastasectomy. Moreover, real-life and interventional studies also suggested that patients who receive bevacizumab with chemotherapy prior to liver resection have improved OS, PFS and metastasectomy rates [24–27]. The favorable impact of bevacizumab in this setting was also found to be associated with reduced sinusoidal obstruction syndrome and hepatic fibrosis [28] as well as enhanced volumetric restoration [29]. This is particularly interesting for chemotherapy-specific liver injuries, which are frequently linked to the use of oxaliplatin in mCRC [30]. Therefore, local ablative treatment for liver metastatic disease seems to be a potential approach to enhance OS for a selected group of patients with mCRC. In addition, we also confirmed the effect of bevacizumab in influencing survival outcomes of mCRC patients with resected primary tumors. In this perspective, a recent meta-analysis of seven studies with 2760 subjects demonstrated that bevacizumab administration to patients with resected primary tumors substantially amplifies the magnitude of OS (HR = 0.50, 95% CI: 0.39–0.64; p < 0.01) and PFS (HR = 0.65, 95% CI: 0.51–0.81; p < 0.01) over no resection or chemotherapy use without bevacizumab [31]. To date, the role of primary tumor resection in the metastatic setting is still debated. Whether such resection and the use of an anti-angiogenic approach can provide survival benefits or not for mCRC patients, this hypothesis should be verified in future trials with adequate sample size. Regarding adverse events of bevacizumab, our results are consistent with the previously reported toxicity profile [17, 32] which included mostly manageable thromboembolic and bleeding events, proteinuria, high blood pressure, and only one serious gastrointestinal perforation.
There is currently an increasingly large body of published evidence suggesting that right- and left-sided CRCs are distinct biological and clinical entities. In addition to the differences in the embryology of these locations – the proximal colon originates from the midgut and the distal colon and the rectum from the hindgut [33] – distinct microbiota profiles were also noted in right- and left-sided CRC [34, 35]. It was demonstrated that this embryological variation leads to a diverse profile in gene expression, methylation, and mutational burden in left- vs. right-sided CRC [36]. In addition, CRC on the right side tends to have mucinous, poorly differentiated, and signet ring histology [37, 38], mutated RAS/RAF and microsatellite instability as compared to the left side, in which CRC is more likely to have altered WNT/MYC signaling and up-regulated CTNNB1 and EGFR/HER2 pathways [38–40]. Tumor sidedness is a well-studied prognostic factor in CRC. Left-sided CRC responds better to chemotherapy and has superior OS as compared to right-sided CRC [38]. Remarkably, the impact of this factor is particularly seen in metastatic disease with respect to response to anti-angiogenics and anti-EGFR blockade [38]. Furthermore, left-sided primary tumor location is associated with a reduced risk of death independently of patients’ race, disease stage and chemotherapy used [41]. These pronounced improved outcomes in patients with left-sided mCRC, as seen in our cohort, were corroborated by multiple randomized clinical trials [42]. Promisingly, a recent meta-analysis of 21 studies demonstrated that bevacizumab-based treatment is superior in terms of efficacy in patients with left-sided mCRC as compared to subjects with right-sided tumors [12], which is in line with the findings of our real-world study. Therefore, this extends the value of tumor sidedness as a possible predictive biomarker of response to bevacizumab. However, this evidence is not sufficiently robust yet to be used in practice for patients’ stratification. From a pathological point of view, the expression of VEGF, the target of bevacizumab, was found to be markedly abundant in the left colon [43, 44]. This may somewhat explain this difference in OS in patients treated with chemotherapy combined with bevacizumab in addition to the previously noted variations.
At present, most of these data supporting the impact of tumor sidedness on survival outcomes and therapy response come from exploratory and post-hoc analyses of clinical trials and poorly designed observational studies, including our real-world investigation. Therefore, the prospective inclusion of this biomarker in future interventional clinical trials with translational genetic and gut microbiome investigations will be fundamental to enhance the quality of the current evidence. The absence of a control group and the molecular profiles of enrolled patients, the relatively small sample size, as well as the retrospective nature of this current report are its principal limitations. However, we believe that these preliminary results are essential to building future projects to improve patients’ care in this setting.
Conclusions
Oxaliplatin and irinotecan-based chemotherapies in combination with bevacizumab are valuable treatment options for patients with left-sided mCRC in medically under-resourced areas with unavailable anti-EGFR targeted agents and RAS tumor profiling. This report raises awareness of the need for molecular pathology in our setting, which is still a neglected area.