Introduction
Sjögren’s syndrome mainly includes primary Sjögren’s syndrome (pSS) and secondary Sjögren’s syndrome (sSS) [1]. sSS usually co-occurs with other autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus (SLE) and scleroderma. pSS is a chronic autoimmune disease, which is characterized by mononuclear cells infiltrating exocrine glands, mainly involving salivary glands and lacrimal glands [2, 3]. The clinical manifestations are dry mouth and dry eyes [4]. Although pSS is more common in exocrine glands, almost all organs may be influenced by pSS. Epidemiology shows that about 60% of pSS patients will have systemic extragonadal involvement [5]. The worrying aspect of Sjögren’s syndrome is that it can induce a variety of complications, such as skin and mucous membrane lesions, nervous system lesions, and digestive system lesions. Among them, interstitial lung disease (ILD) is one of the most common serious complications, which can lead to infection and respiratory failure, and is the main cause of poor prognosis in patients with pSS [6]. In addition, pSS also has immune complex-mediated characteristics, including salivary gland hypertrophy, cryoglobulinemia, and hypocomplementemia [7]. Among them, hyper-gamma-globulinemia is the most serious extra-glandular organ complication, which has a high risk of developing into malignant lymphoma [8]. Therefore, early diagnosis and risk assessment of pSS are of great clinical significance.
The chemokine (C-X-C motif) ligand 13 (CXCL13), also known as B-lymphocyte chemokine, is localized on chromosome 4q21, and is mainly distributed in human liver, serum, lymph nodes and stomach [9, 10]. Studies have found that high levels of CXCL13 were associated with pSS and SLE. A previous related study showed that in pSS patients with high ESSDAI scores and B-cell activation, the serum CXCL13 levels were significantly increased [11]. Macrophage migration inhibitory factor (MIF) is a pleiotropic immunoregulator factor with a unique structure, which mediates host responses to infection and stress through innate and adaptive immune pathways [12]. It is found that MIF is significantly associated with the development of autoimmune diseases (rheumatoid arthritis, juvenile idiopathic arthritis) [13]. Several studies have reported that elevated MIF levels have been detected in blood, saliva and urine of pSS patients [14, 15]. In recent years, cytokines have played a very important role in the occurrence and development of pSS. Interleukin 35 (IL-35) is an anti-inflammatory cytokine that was discovered by Collison in 2007. Like other members of the IL-12 family, IL-35 is an irritating cytokine composed of p35 and EBI3 subunits, that is, a heterodimer, so it has the commonness of the IL-12 family [16]. A study showed that the content of IL-35 increases in SLE, and can effectively inhibit the expression level of inflammatory factors in SLE [17]. In another study, it was observed that the existence of IL-35 could effectively inhibit the proliferation of synovial cells and the destruction of adjacent cartilage in a mouse arthritis model [18]. However, the relationship between CXCL13, MIF, and IL-35 and the severity of pSS is not clear.
This study intends to evaluate the interplay between factors such as CXCL13, MIF and IL-35 and pSS by measuring the levels of CXCL13, MIF and IL-35 in blood samples of pSS patients.
Material and methods
Study population and sample collection
A total of 133 patients with pSS diagnosed in the Second Hospital of Hebei Medical University were included in this study. Among them, there were 119 females and 14 males, aged from 18 to 82 years. Inclusion criteria: pSS cases were diagnosed according to the International Classification (Diagnostic) criteria for Sjögren’s syndrome in 2002 [19]. Also, according to the presence or absence of interstitial lung disease (ILD), the patients were divided into the pSS group and the PSS-ILD group. Exclusion criteria: 1) smoking; 2) those who had used antibiotics, antifungal drugs, immunosuppressants and other drugs in the 2 weeks prior to enrollment; 3) history of head and neck radiotherapy and chemotherapy; 4) patients with sarcoidosis, hepatitis C, amyloidosis, acquired immunodeficiency syndrome (AIDS), graft-versus-host disease, IgG4-related diseases and lymphoma. Sixty-two healthy adults who underwent physical examination in this hospital during the same period were selected as the control group, and were age and sex matched. All participants signed an informed consent form, and this study was approved by the Medical Ethics Committee of the Second Hospital of Hebei Medical University. After all the subjects were enrolled in the group, 3 ml of saliva was taken the next morning and stored in a low-temperature refrigerator at –80oC for further use.
Evaluation of primary Sjögren’s syndrome activity
European League Against Rheumatism (EULAR) Sjögren’s syndrome disease activity index (ESSDAI) was used to evaluate the disease activity of patients with pSS. The ESSDAI score is one of the most objective and comprehensive scoring criteria to evaluate pSS activity, and it is assigned according to the pathological degree of 12 aspects: lymphadenopathy, general condition, gland involvement, joint abnormality, skin appearance, muscle involvement, urinary system, respiratory system, peripheral nervous system, blood system, central nervous system and biological indicators [20].
Enzyme linked immunosorbent assay
The levels of CXCL13, MIF and IL-35 in saliva samples were detected by enzyme linked immunosorbent assay (ELISA). The absorbance values of each group were detected using a Bio-RAD680 microplate reader, and the operation was carried out according to the instruction manual. The commercially available ELISA kits (CusaBio, Wuhan, China) used in this study are as follows: CXCL13 (Human ELISA kit, catalogue number: CSB-E16832m), MIF (Human ELISA kit, catalogue number: CSB-E08330h), IL-35 (Human ELISA kit, catalogue number: CSB-E13126h).
Statistical analysis
SPSS 21.0 was used for statistical analysis. Measurement data were normally distributed and expressed as mean ± standard deviation. The chi-square test was used to compare categorical variables. T-test and one-way ANOVA were used to compare two or more groups. Logistic regression was used to evaluate the relationship between different variables and the development of pSS into pSS-ILD. Pearson correlation analysis was used to evaluate the relationship between serum CXCL13, MIF, IL-35 levels and ESSDAI score in all patients. The receiver operating characteristic (ROC) curve was used to evaluate the discriminative ability of CXCL13, MIF, and IL-35 for pSS-ILD in pSS. P < 0.05 was considered statistically significant.
Results
Comparison of baseline data
As shown in Table 1, there were no significant differences in age, gender, or body mass index (BMI) in the three groups (all p > 0.05). Notably, the ESR level in the pSS group was higher than that in the control group (p < 0.001), while the ESR level in the pSS-ILD group was higher than that in the pSS group (p < 0.001). Meanwhile, the ESSDAI score of the pSS-ILD group was higher than that of the pSS group (p < 0.001). There was no difference in the level of IgG between the pSS group and PSS-ILD group (p > 0.05), but the levels of IgG in both groups were significantly higher than those in the control group (p < 0.001). Furthermore, there was no significant difference in the case number of dry mouth and dry eye between the pSS group and the pSS-ILD group (p > 0.05).
Table 1
Comparison of baseline clinical data in three groups
[i] pSS – primary Sjögren’s syndrome, ILD – interstitial lung disease, BMI – body mass index, ESR – erythrocyte sedimentation rate, ESSDAI – European League Against Rheumatism (EULAR) Sjögren’s syndrome disease activity index, IgG – immunoglobulin G, anti-SSA – anti-Sjögren’s syndrome A, anti-SSB – anti-Sjögren’s syndrome B, ANA – antinuclear antibody, NA – not applicable.
CXCL13, MIF, and IL-35 levels in saliva
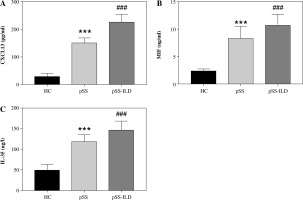
The concentrations of CXCL13, MIF, and IL-35 were detected by ELISA. As shown in Figure 1A-C, compared with the healthy controls, all the index values in the case group showed a significant upward trend (p < 0.001). The levels of CXCL13, MIF and IL-35 were obviously higher in the PSS-ILD group than the pSS group (p < 0.001).
Fig. 1
Saliva expression of CXCL13, MIF, IL-35. A) Saliva expression of CXCL13 in healthy controls, pSS group, and pSS-ILD group. B) Saliva expression of MIF in healthy controls, pSS group, and pSS-ILD group. C) Saliva expression of IL-35 in healthy controls, pSS group, and pSS-ILD group. ***p < 0.001 vs. HC, ###p < 0.001 vs. pSS group
Correlation analysis between CXCL13, MIF, IL-35 and ESSDAI
As illustrated in Table 2, Pearson’s correlation coefficient analysis showed that ESSDAI score was positively correlated with the levels of CXCL13 (r = 0.687, p < 0.001), MIF (r = 0.511, p < 0.001), and IL-35 (r = 0.602, p < 0.001), respectively.
Risk factors for conversion of pSS to pSS-ILD
Logistic regression analysis was used to evaluate the effect of each index on the transformation from pSS to pSS-ILD. As shown in Table 3, after excluding age and other confounding factors, CXCL13 (OR = 0.185, 95% CI = 0.082-0.417, p < 0.001) and IL-35 (OR = 0.407, 95% CI = 0.176-0.891, p = 0.023) were found to be independent risk factors for the development of pSS to pSS-ILD.
Table 3
Relationship between different variables and the development of pSS into pSS-ILD
[i] pSS – primary Sjögren’s syndrome, ILD – interstitial lung disease, BMI – body mass index, ESR – erythrocyte sedimentation rate, IgG – immunoglobulin G, ESSDAI – European League Against Rheumatism (EULAR) Sjögren’s syndrome disease activity index, CXCL13 – chemokine (C-X-C motif) ligand 13, MIF – macrophage migration inhibitory factor, IL-35 – interleukin-35, OR – odds ratio, 95% CI – confidence interval
ROC analysis
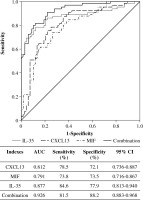
ROC curve analysis was used to evaluate the ability of CXCL13, MIF, IL-35, and their combination to distinguish patients with PSS-ILD from pSS. As shown in Figure 2, the AUC value of the combined detection curve of the three indexes was 0.926, and the sensitivity and specificity were 81.5% and 88.2%, respectively, which are better than those of other indexes separately.
Discussion
The pathogenic factors and pathogenesis of pSS are still unclear, but it is generally believed that it may be related to viral infection, abnormal sex hormones and genetic factors. Under the joint action of pathogenic factors, the organism’s autoimmune disorder and cellular and humoral immunity are abnormal. These changes promote the organism to synthesize and secrete a variety of inflammatory mediators and cytokines, thus damaging plasma cells and lymphocytes, and then damaging skin, the lacrimal gland, liver, kidney, lung and other tissues and organs [21, 22]. At present, the main clinical diagnosis of pSS is based on serum anti-SSA (anti-Sjögren’s syndrome A), and anti-SSB (anti-Sjögren’s syndrome B) antibodies. These two antibodies are highly specific in the diagnosis of pSS. Anti-SSB anti-bodies often coexist with anti-SSA antibodies, which are more specific than anti-SSA antibodies in diagnosing pSS. Anti-SSA antibodies are directly involved in histopathological lesions, especially skin lesions, which can lead to subacute cutaneous lupus and neonatal lupus syndrome [23]. However, the positive rates of anti-SSA/SSB antibodies are very high in many autoimmune diseases (vasculitis, leukopenia, photoallergy, and skin lesions), and pSS is only one of the autoimmune diseases [24]. At present, there is a lack of specific indicators for the diagnosis of pSS. Therefore, it is crucial to explore other related indicators to improve the sensitivity and specificity of pSS diagnosis.
In this study, saliva of patients was collected to detect the relevant indicators. The main advantages of saliva samples are non-invasiveness, ease of collection, and patient acceptance. Therefore, saliva marker examination plays an increasingly important role in the clinical evaluation of pSS, and it is expected to be one of the important indicators for early diagnosis, screening, or disease monitoring of pSS. The results of this study showed that the salivary CXCL13, MIF and IL-35 levels of pSS patients were significantly higher than those of healthy people. However, the levels of CXCL13, MIF and IL-35 in the pSS-ILD group were higher than those in the pSS group. Accordingly, it is reasonable to suspect that these three indicators are related to the severity of pSS. Previously, Kramer et al. reported that the expression of CXCL13 was elevated in blood and saliva of human pSS patients and mouse models of pSS, and the level of CXCL13 increased continuously with the progress of the disease [25]. As reported by Willeke et al., they found that the MIF in the blood of patients with pSS has an upward trend [13]. Additionally, the study of Han et al. confirmed that the expression of IL-35 is enhanced in pSS patients [26]. This evidence suggests that CXCL13, MIF and IL-35 may play an important role in the diagnosis, screening or disease monitoring of pSS.
In 2009, the European League on Rheumatism (EULAR) organized more than 30 experts to formulate the ESSDAI score [27]. The ESSDAI assessment system mainly includes 12 aspects: systemic symptoms, lymph nodes, glands, joints, skin, lung, kidney, skeletal muscle, peripheral nervous system, central nervous system, blood system and serological changes [28]. However, the evaluation process of this index requires professional and experienced clinicians to carry out the evaluation in strict accordance with the complex project with certain human factors. In the current study, all patients with pSS were evaluated in a standardized manner based on the standard ESSDAI assessment. The experimental results showed that the ESSDAI score was significantly positively correlated with the levels of CXCL13, MIF and IL-35, which also objectively proved again that CXCL13, MIF, and IL-35 were related to disease activity. After excluding the influence of gender and other confounding factors, logistic regression analysis showed that CXCL13 and IL-35 were independent risk factors for pSS to develop into pSS-ILD. At present, there are many reports on the application of serum CXCL13, MIF and IL-35 in the diagnosis or prognosis of pSS, but there are few studies on the combined detection of these three indicators. In this study, the accuracy of the combined detection of CXCL13, MIF and IL-35 for distinguishing pSS-ILD from pSS was higher than the sensitivity and specificity of using the three indicators alone. This analysis shows that the combined detection of multiple indicators can be used as a good indicator for disease screening, and for disease diagnosis, the combined detection may greatly improve the diagnostic accuracy of disease.
Our study has some limitations. On the one hand, the sample size in the study cohort is relatively small, and all samples come from the same hospital, so selection bias may not be ruled out. On the other hand, CXCL13, MIF and IL-35 were detected from saliva samples rather than blood. Although it has been reported that these three indicators can be detected in human blood, saliva, and urine, we cannot explain whether the expression trends of these three indicators in blood, saliva and urine are consistent. Therefore, in subsequent experiments, multi-center cohorts with a large sample size should be selected as far as possible for relevant detection. At the same time, blood is one of the important body fluids, and the detection of biomarkers in blood may be more attractive than in other body fluids.
In summary, the expression levels of CXCL13, MIF and IL-35 in saliva of pSS patients increased, and were positively correlated with ESSDAI score. In addition, CXCL13 and IL-35 are independent risk factors for the development of pSS to pSS-ILD, and the sensitivity and specificity of the combination of the three indicators for distinguishing pSS-ILD from pSS are higher than those when the three indicators are used alone.